Iminium salts and methods of preparing electron deficient olefins using such novel iminium salts
An iminium salt, atomic technology, applied in the field of new iminium salt, can solve the problem of no iminium salt and/or ionic liquid application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
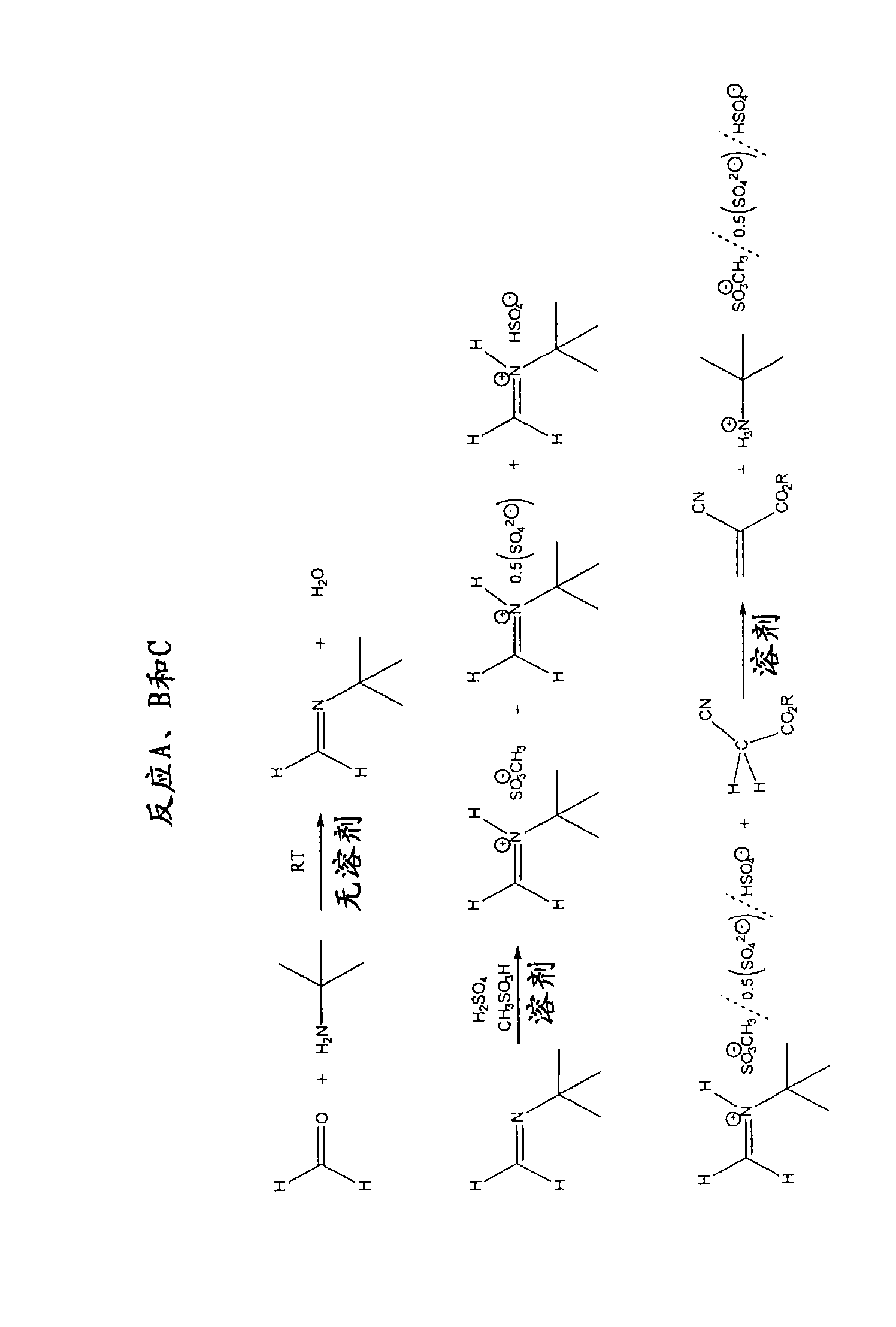
PRIMENE imine ( figure 2 ,step 1)
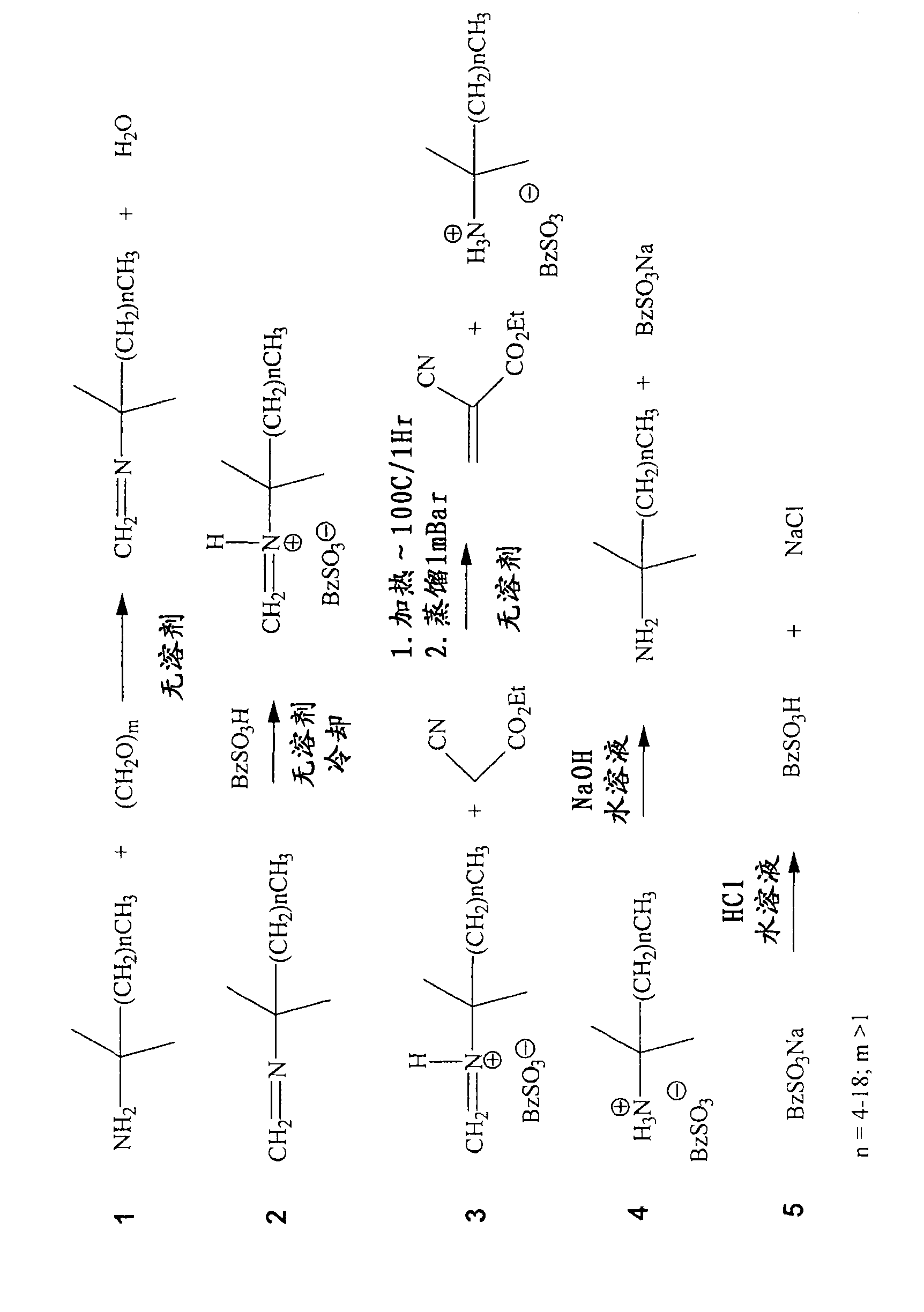
[0074] Imines were prepared using PRIMENE TOA, 81-R and JM-T by reacting amines with a stoichiometric equivalent of paraformaldehyde and removing the water of condensation. All imines formed were distillable liquids and existed as stable monomeric imines as demonstrated by: 1 HNMR 60MHz (CDCl 3 ) 2H D 7.16, 7.09ppm (TOA imine); 2H s (br) 7.45ppm (81-R imine) and (CD 3 COCD 3 )2H 6.86PPM (JM-T imine) and FTIR (1650cm for each -1 ).
PRIMENE iminium salt ( figure 2 , step 2)
[0075] The distilled imine was analyzed by Karl-Fischer titration and appeared to be essentially free of water (<20 ppm). These imines were treated with a stoichiometric equivalent of acid while cooling. Iminium salts are prepared from the following acids: methanesulfonic acid, benzenesulfonic acid, sulfuric acid and mixtures of some of these.
[0076]PRIMENE TOA iminium methane sulfonate forms a viscous liquid that begins to show signs of crystallization af...
Embodiment 2
[0084] In this example, various amines were synthesized and characterized using the following instruments: JNM-MY60 (60MHz for 1 hour) or Varian UNITY-300 (for 1 H is 300MHz, and for 13 C NMR was recorded at 75.5 MHz). Chemical shifts δ are given in ppm relative to the residual peak of the deuterated solvent and coupling constants J are given in Hertz.
[0085] All amines (PRIMENE TOA, 81-R, JMT, MD from Rohm and Haas), acids (methanesulfonic acid, acetic acid, p-toluenesulfonic acid, benzenesulfonic acid, sulfuric acid and phosphoric acid from Aldrich), solvents ( Toluene, benzene, ether, heptane, hexane, methylene chloride, chloroform from Aldrich), n-butyl lactoyl cyanoacetate (from Degussa), other chemicals (cyanoacetic acid, cyanide from Aldrich Ethyl acetate, 2-hydroxyethyl acrylate, 2-hydroxyethyl methacrylate, hydroxypropyl acrylate, hydroxypropyl methacrylate) can be directly purchased and used without further refining.
[0086] The corresponding PRIMENE imines are...
Embodiment 3
[0093] In this example, various iminium salts were synthesized from the imines prepared above.
Synthesis of PRIMENE 81-R iminium-MSA
[0095] Methanesulfonic acid (96 g, 1.0 mol) was added dropwise to freshly distilled PRIMENE 81-R imine (197 g, ~1.0 mol) with stirring at ice-water bath temperature and then warmed to room temperature. The pale yellow iminium salt formed was retained for further reaction.
Synthesis of other iminium salts
[0096] Follow the same procedure to prepare the following iminium salts:
Table 1
Note: MSA = methanesulfonic acid; BSA = benzenesulfonic acid; LAS = dodecylbenzenesulfonic acid, NSA = 2-naphthalenesulfonic acid; s = solid; √ = ionic liquid.
*Using 2 molar equivalents of acid.
[0097] Ethyl cyanoacrylates and other electron-deficient alkenes were synthesized from various iminium salts using the following general conditions. Ethyl cyanoacetate (1.0 eq) and iminium salt (1.0 eq) were mixed together with stirring at 100 °C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com