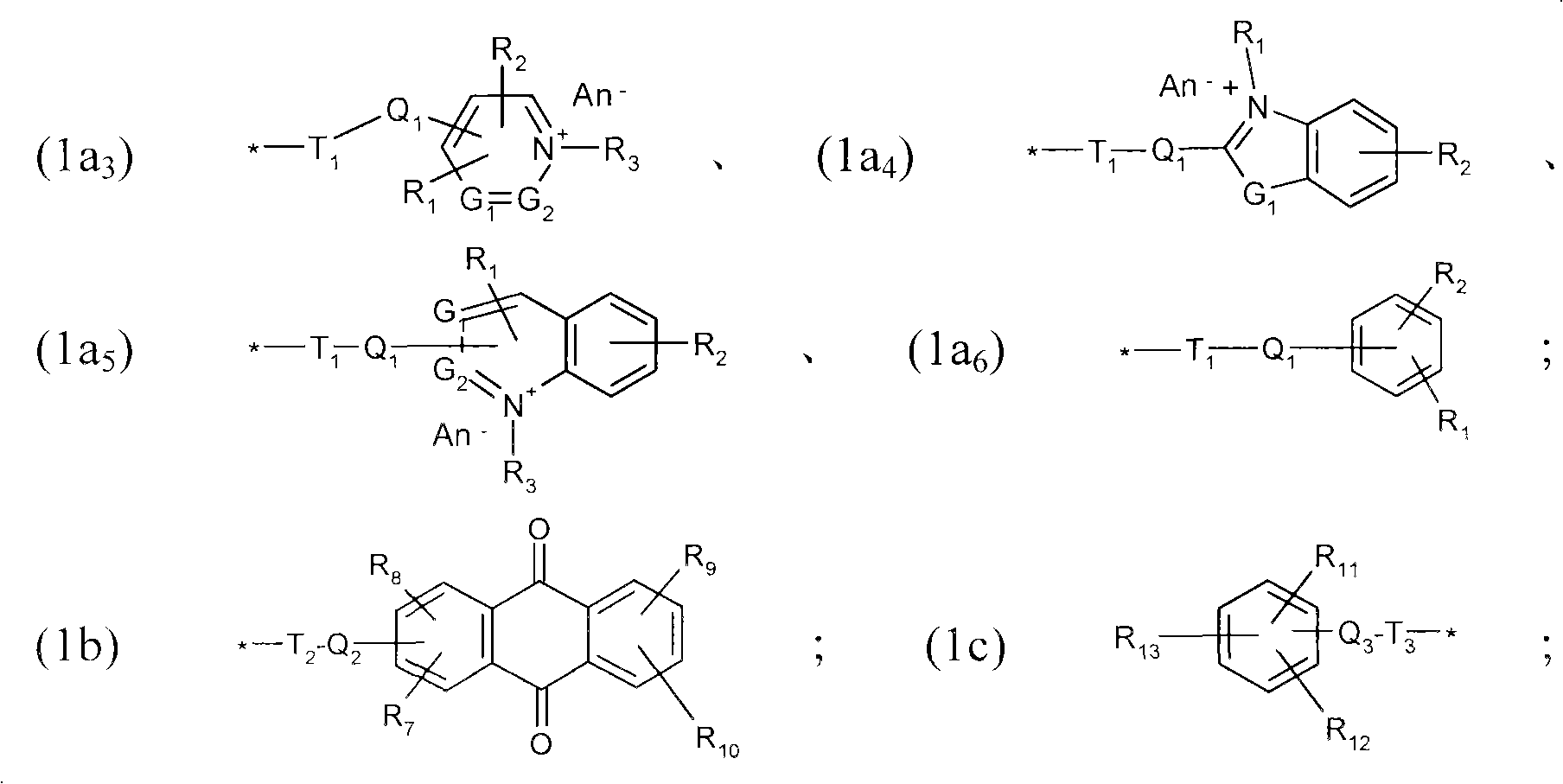
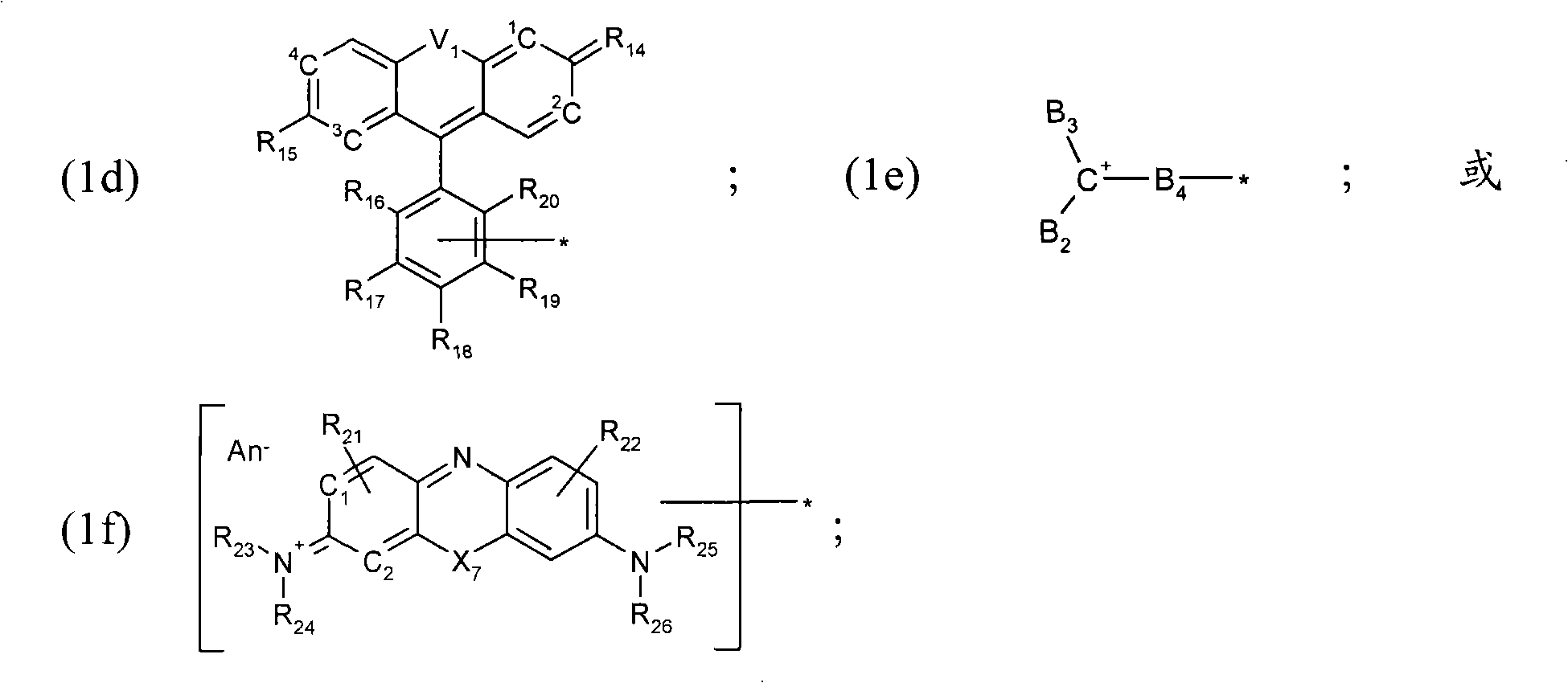
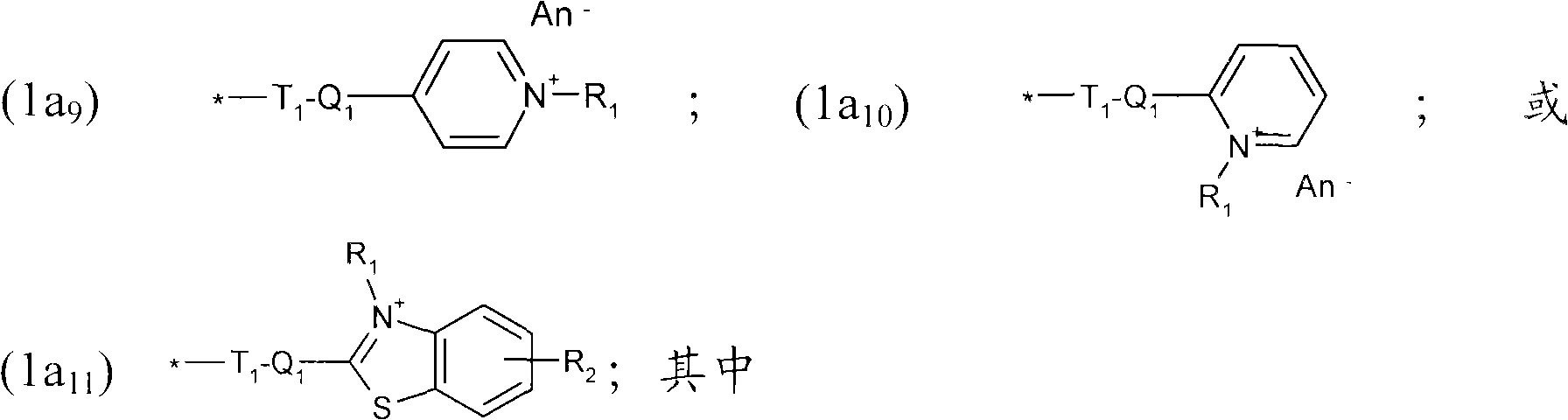Thiol derivative dyes
A compound, alkyl technology, applied in the direction of sulfur dyes, azo dyes, organic dyes, etc., can solve the problem of unsatisfactory washing fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A-1
[0379] Example A-1: Preparation of compound of formula (101)
[0380]
[0381] A-1.1 Monoazo (monoazo)
[0382] At 295K, 62 g of 4-fluoroaniline was added to a stirred solution of 125 ml of water and 12 ml of 32% hydrochloric acid. Then, the reaction mixture was cooled to 273K and 95 ml of 36% sodium nitrite solution was added dropwise to control the dropping rate so that the temperature of the mixture was maintained at 273 to 276K. After adding the sodium nitrite solution, the mixture was stirred for one hour. If no remaining nitrite is detected within one hour (use potassium iodide test paper for detection), then add a certain amount of sodium nitrite solution. After this hour, the remaining excess nitrite was reduced with sulfamic acid. Then, the obtained diazo-solution was added dropwise to a 273K cold solution of 38 g of imidazole in 30 ml of water, thereby maintaining the pH of the solution in the range of pH 10 to 11 by adding 36% sodium hydroxide solution. After the a...
Embodiment A-2
[0397] Example A-2: Preparation of the compound of formula (102)
[0398]
[0399] A-2.1 Alkylation
[0400] One equivalent (6.0 g) of thiourea was dissolved in 30 g of the aforementioned alkylating dye solution in absolute ethanol. The temperature was raised to reflux and the temperature was maintained at 80°C for the next 48 hours.
[0401] A-2.2 Hydrolysis
[0402] One equivalent (4.0 g) of sodium hydroxide was dissolved in the absolute ethanol solution of the aforementioned substances. The temperature was maintained at 80°C for the next 4 hours. Under stirring, the product was crystallized by cooling to room temperature, and then separated by filtration, washed and dried in a vacuum dryer.
[0403] Use the following data to characterize the product:
[0404] In deuterated chloroform 1 H-NMR data (128 scans) / 360MHz
[0405] 8.003 d 6.7 2.00 Phenyl
[0406] 7.576 s 2.01 imidazolyl
[0407] 7.000 d 6.9 2.01 phenyl
[0408] 4.060 s 6.00 methyl
[0409] 4.002 t 6 2.03 ethyl...
Embodiment A-3
[0412] Example A-3: Preparation of the compound of formula (103)
[0413]
[0414] A-3.1 Alkylation
[0415] One equivalent (10.0 g) of potassium thioacetate was dissolved in a solution of 30 g of the aforementioned alkylated dye in 75 ml of ethanol. The temperature was raised to reflux and the temperature was maintained at 80°C for the next 4 hours.
[0416] Use the following data to characterize the product:
[0417] In deuterated chloroform 1 H-NMR data (128 scans) / 360MHz
[0418] 8.003 d 6.7 2.00 Phenyl
[0419] 7.578 s 2.01 imidazolyl
[0420] 7.060 d 6.9 2.01 phenyl
[0421] 4.074 s 6.00 methyl
[0422] 3.352 t 6 2.03 ethylene
[0423] 3.085 t 6 2.05 ethylene
[0424] 2.289 s 3.087 acetate
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com