Method for separating dichlorohydrin from compound
A technology of dichloropropanol and mixture, applied in the field of preparation of epichlorohydrin, can solve the problems of increasing reaction unit consumption, polluting the environment, large amount of waste water, etc., achieving high recovery rate, reducing environmental pollution and increasing production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
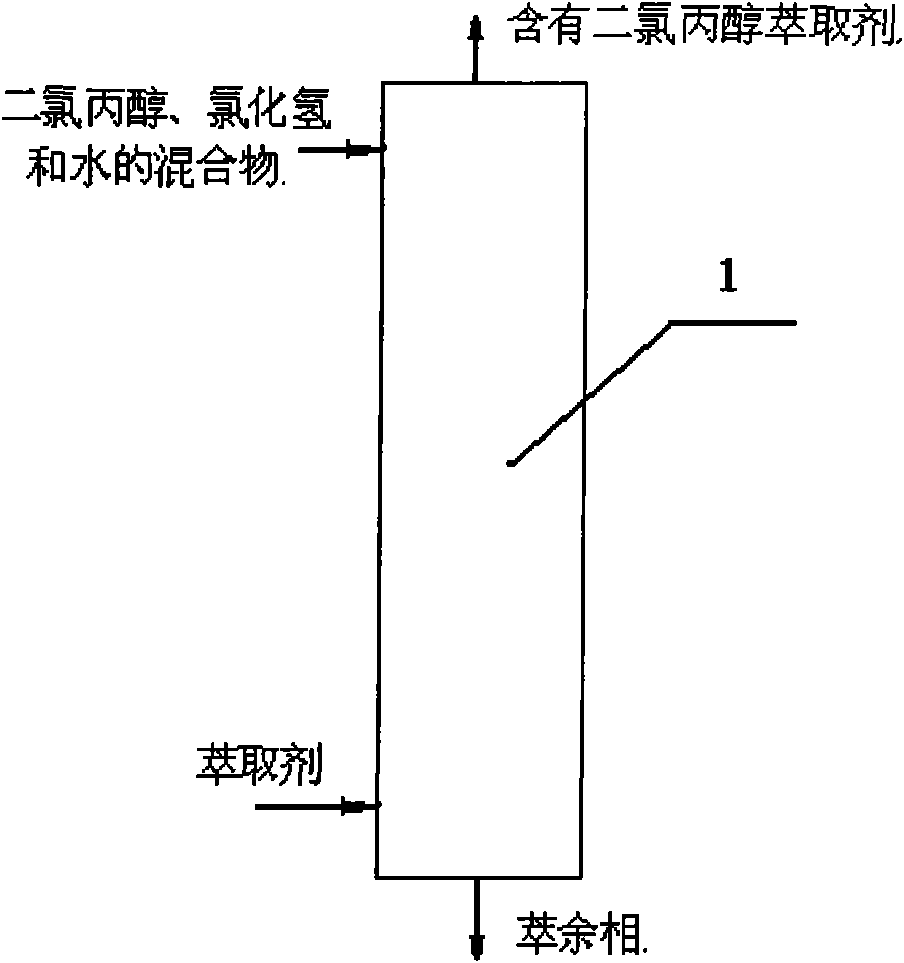
[0025] Extraction tower: sieve plate tower with 3 balance stages.
[0026] The mixture of dichloropropanol, hydrogen chloride and water is sent into the extraction tower 1 from the upper part, and the extractant is sent into the extraction tower 1 from the lower part for countercurrent extraction, and the extractant containing dichloropropanol is discharged from the top of the extraction tower and sent to the subsequent The rectifying tower, the raffinate phase is discharged from the bottom of the extraction tower 1.
[0027] In the mixture, the weight content of 1,3-dichloro-2-propanol is 40.2%, the weight content of 1,2-dichloro-3-propanol is 5%, and in the mixture, the weight content of hydrogen chloride is 27%, The weight content of water is 27.8%;
[0028] The extractant is n-octanol; the weight ratio of the mixture of dichloropropanol, hydrogen chloride and water to the extractant is:
[0029] The mixture of dichloropropanol, hydrogen chloride and water: extractant=1: ...
Embodiment 2
[0033] Extraction tower: It adopts a rotating disk tower with 5 balance stages.
[0034] The mixture of dichloropropanol, hydrogen chloride and water is sent into the extraction tower 1 from the upper part, and the extractant is sent into the extraction tower 1 from the lower part for countercurrent extraction, and the extractant containing dichloropropanol is discharged from the top of the extraction tower and sent to the subsequent The rectifying tower, the raffinate phase is discharged from the bottom of the extraction tower 1.
[0035] In the mixture, the weight content of 1,3-dichloro-2-propanol is 40.2%, the weight content of 1,2-dichloro-3-propanol is 5%, and in the mixture, the weight content of hydrogen chloride is 27%, The weight content of water is 27.8%;
[0036] Described extractant is n-butyl ether;
[0037] The weight ratio of the mixture of dichloropropanol, hydrogen chloride and water to the extractant is:
[0038] The mixture of dichloropropanol, hydrogen ...
Embodiment 3
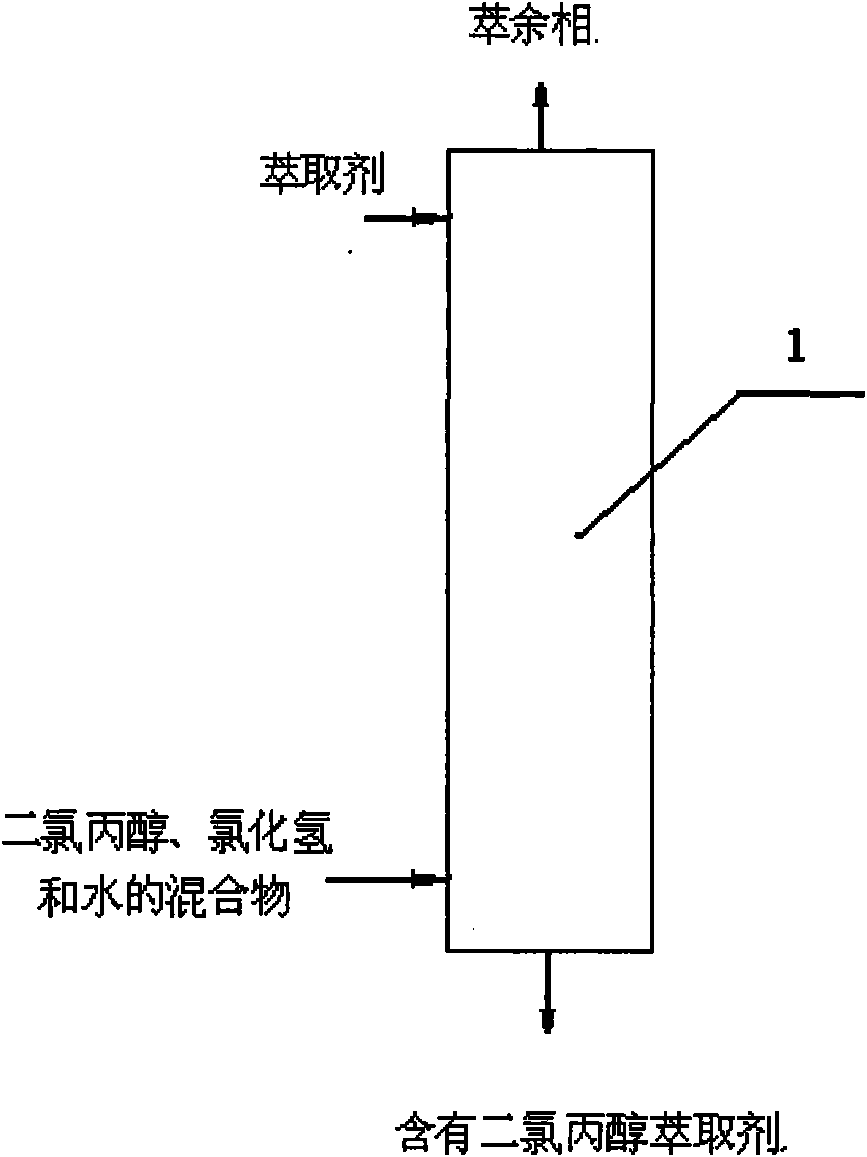
[0042] use figure 2 process.
[0043] Extraction tower: It adopts a rotating disk tower with 5 balance stages.
[0044] The mixture of dichloropropanol, hydrogen chloride and water is sent into the extraction tower 1 from the lower part, and the extractant is sent into the extraction tower 1 from the upper part for countercurrent extraction, and the extractant containing dichloropropanol is discharged from the top of the extraction tower and sent to the subsequent The rectifying tower, the raffinate phase is discharged from the top of the extraction tower 1.
[0045] In the mixture, the weight content of 1,3-dichloro-2-propanol is 40.2%, the weight content of 1,2-dichloro-3-propanol is 5%, and in the mixture, the weight content of hydrogen chloride is 27%, The weight content of water is 27.8%;
[0046] The extractant is 1,2-dichloroethane;
[0047] The weight ratio of the mixture of dichloropropanol, hydrogen chloride and water to the extractant is:
[0048] The mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com