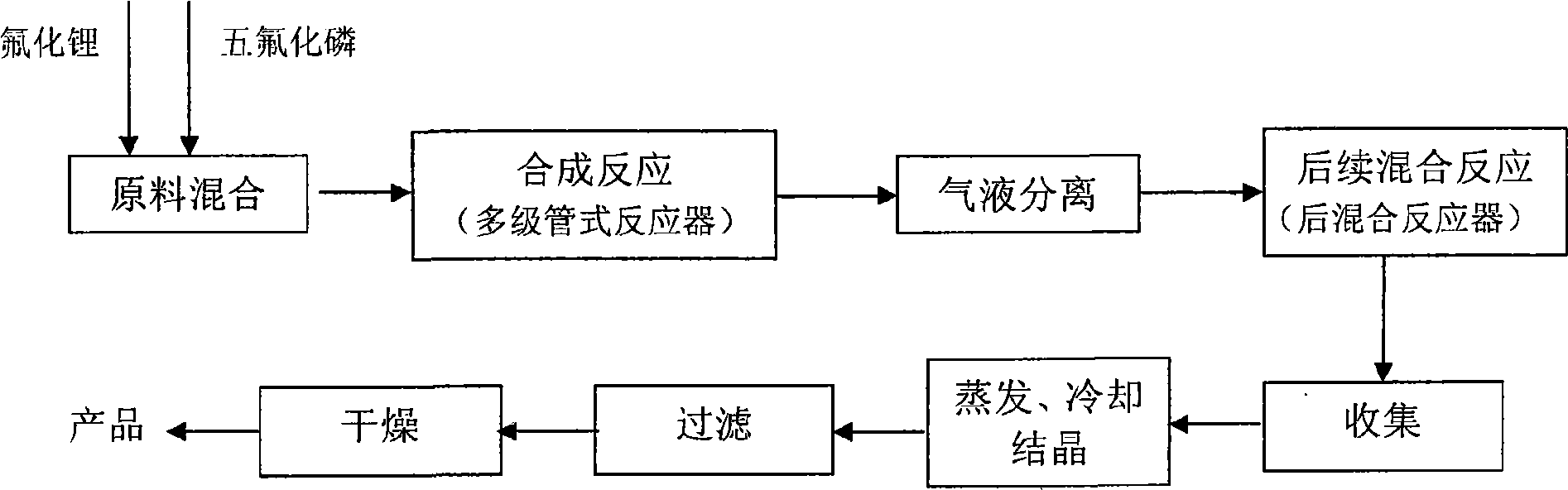
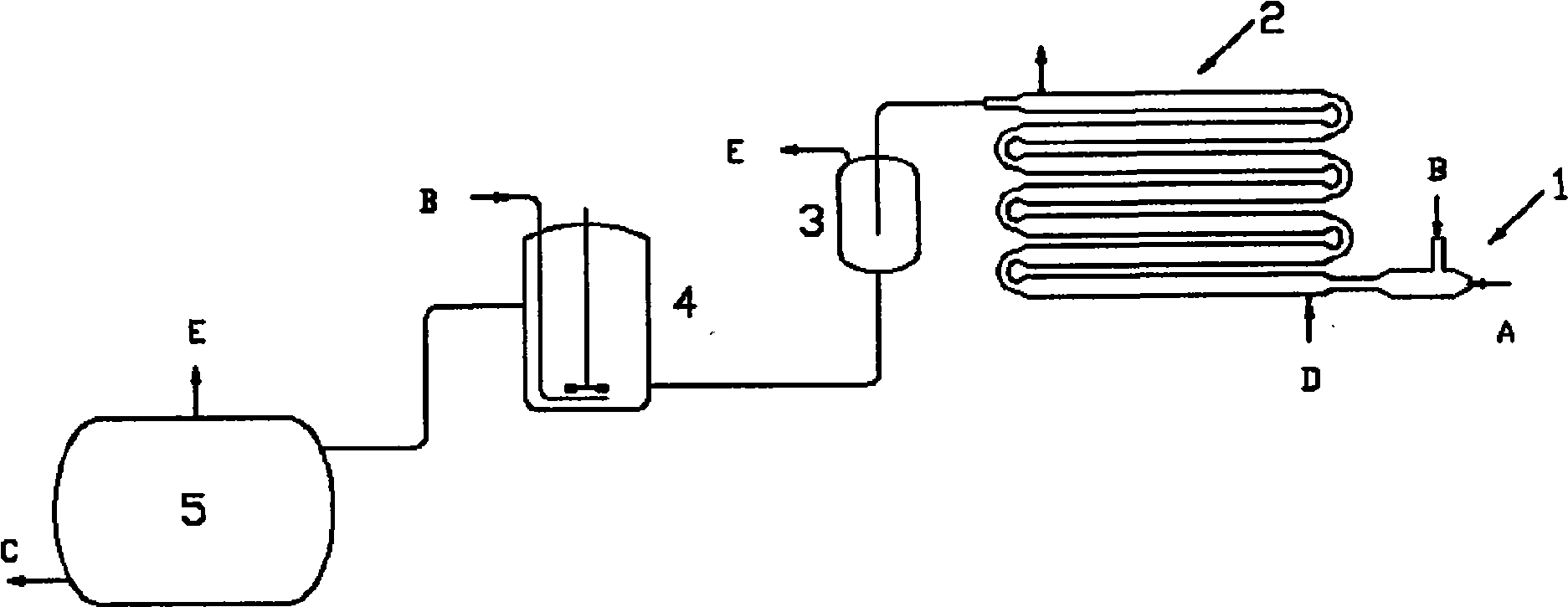
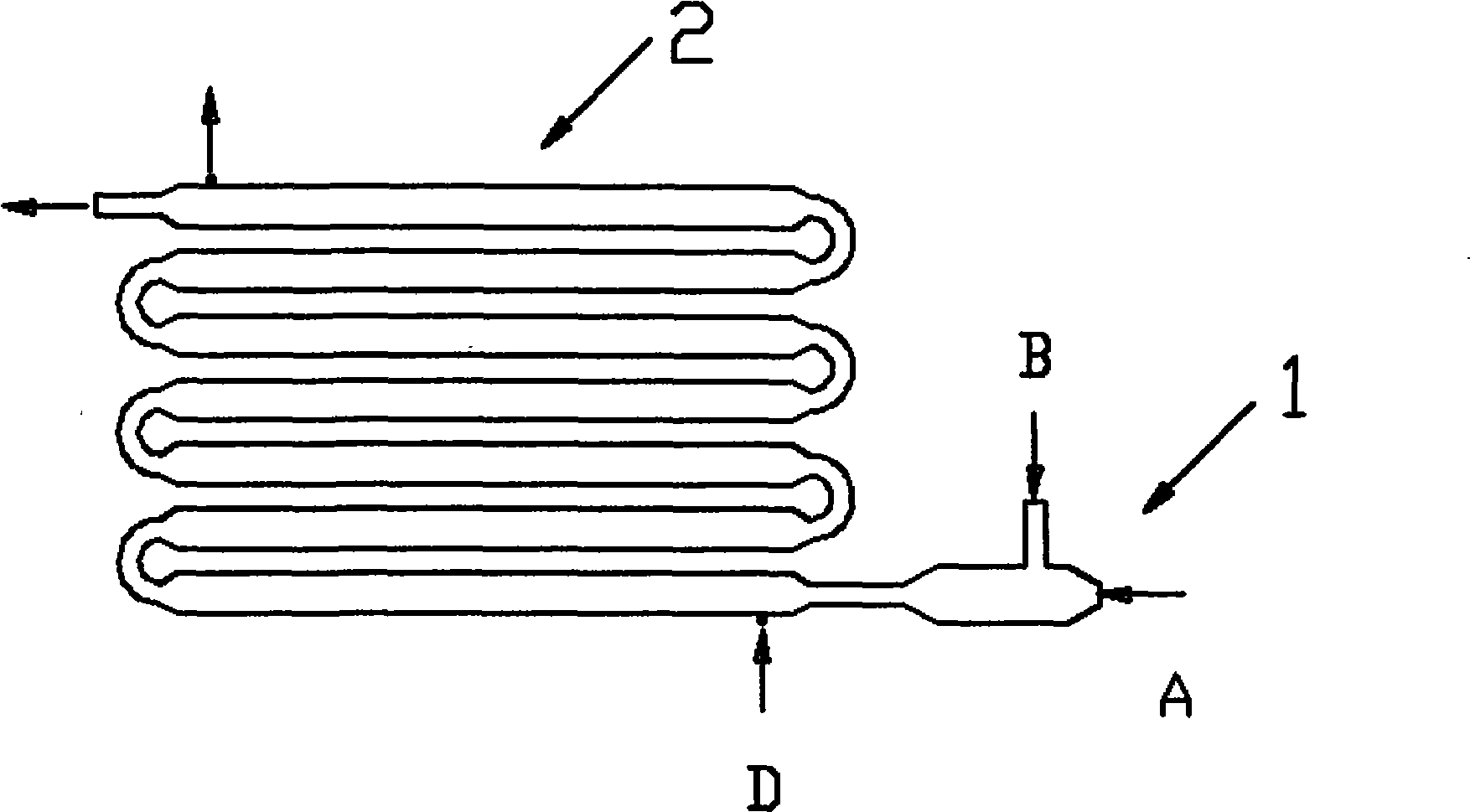
Process and device for continuous preparation of lithium hexafluorophosphate
A technology of lithium hexafluorophosphate and preparation process, which is applied in phosphorus compounds, inorganic chemistry, electrochemical generators, etc., can solve problems such as non-continuous production, and achieve the effect of achieving continuity and reducing equipment investment and production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Such as figure 1 , shown in 2, the present embodiment is carried out in a multi-stage tubular reactor, and the effective reaction volume is 10L. A compressor is used to send gaseous reactants from the reactor inlet into the reactor, and the reactants have the following composition:
[0047] PF5----98.5%
[0048] Inert gas----1.1%
[0049] The above content is the molar content.
[0050] The total flow rate is 26.5 mol / hr, and the converted flow rate of phosphorus pentafluoride is 26.1 mol / hr, that is, 3290 grams per hour. At the same time, anhydrous hydrogen fluoride solution containing lithium fluoride is pumped into as liquid reactant, and the solution has the following composition:
[0051] LiF ---- 3.0%
[0052] HF----97%
[0053] The above content is the mass content.
[0054] The total flow rate of liquid reactants is 20kg / hr, of which 600g / hr of lithium fluoride is equivalent to 23.1mol / hr. Therefore, the molar ratio of phosphorus pentafluoride to lithiu...
Embodiment 2
[0058] Take the mother liquor after crystallization and filtration in Example 1, and add purified lithium fluoride under stirring to form a solution. The content of lithium fluoride in the solution is 3.06%, and the rest is anhydrous hydrogen fluoride and dissolved lithium hexafluorophosphate. This solution was used as the liquid reactant in the tubular reactor.
[0059] A compressor is used to send the gaseous reactants into the reactor from the reactor inlet, and the composition of the gaseous reactants is exactly the same as in Example 1.
[0060] The total flow rate is 26.5 mol / hr, and the converted flow rate of phosphorus pentafluoride is 26.1 mol / hr, that is, 3290 grams per hour.
[0061] The total flow rate of liquid reactants is 20g / hr, of which 612g / hr of lithium fluoride is equivalent to 23.5mol / hr.
[0062] Therefore, the molar ratio of phosphorus pentafluoride to lithium fluoride is about 1.11.
[0063] The above gas and liquid reactants react in the tubular rea...
Embodiment 3
[0066] Multi-stage synthesis reaction: the mixed gas and solution enter the multi-stage tubular reactor through the gas-liquid mixer with the molar ratio of phosphorus pentafluoride and lithium fluoride at a ratio of 1 to 5:1, and the reaction is carried out at a temperature of -40°C ~ 30°C, reaction pressure -0.5 ~ 1.5MPa, after multi-stage full reaction, synthesize lithium hexafluorophosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com