Preparation method of ulifloxacin optical isomer
A technology of optical isomers and structural formulas, used in organic chemistry, anti-infective drugs, drug combinations, etc., can solve problems such as low yield, inability to apply industrial-scale production, and large equipment investment, achieve high purity, and facilitate industrial mass production. , the effect of the simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
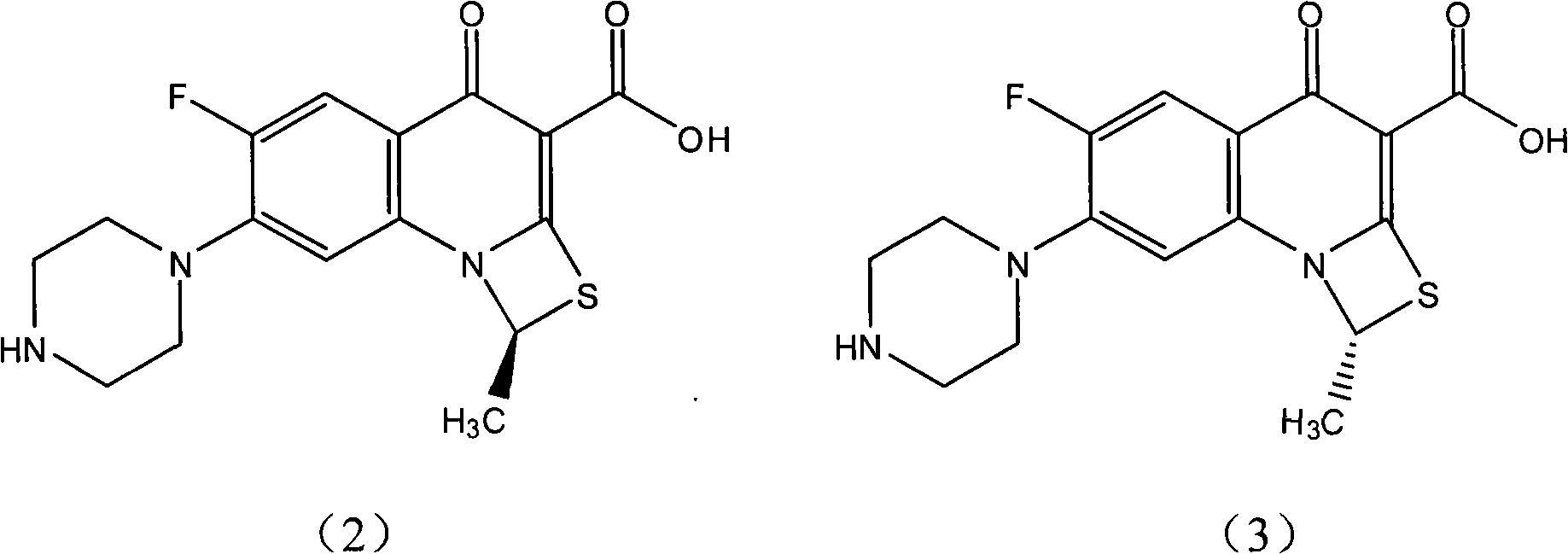
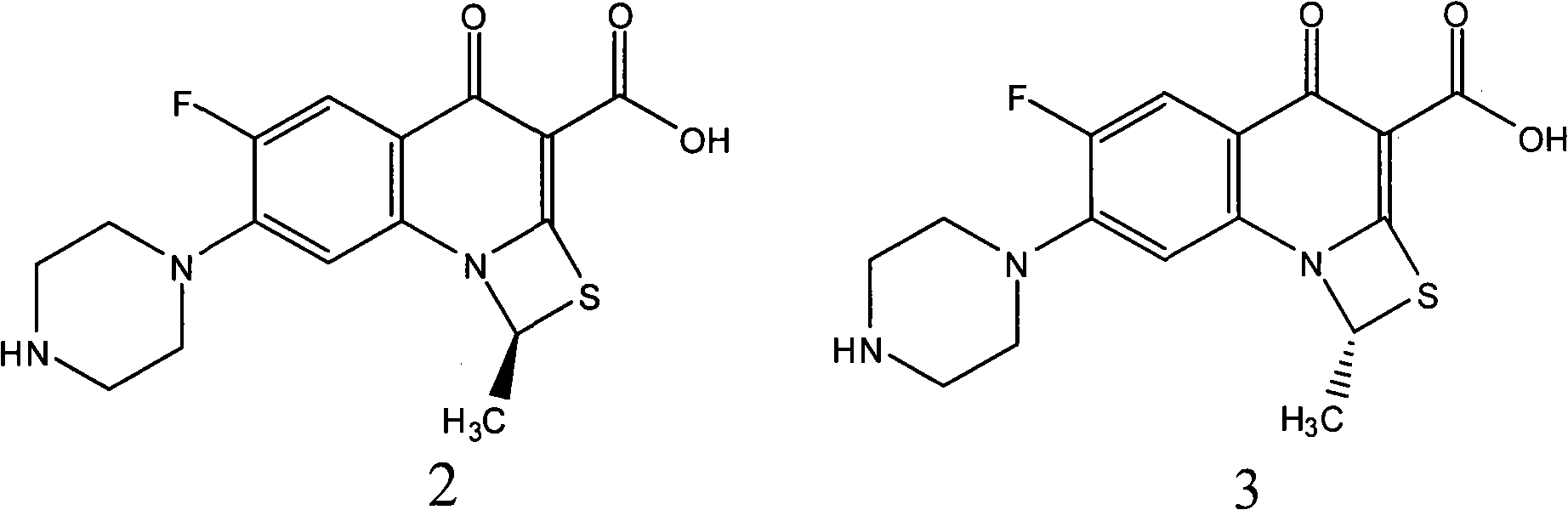
[0011] The preparation of embodiment 1 (S)-ulifloxacin
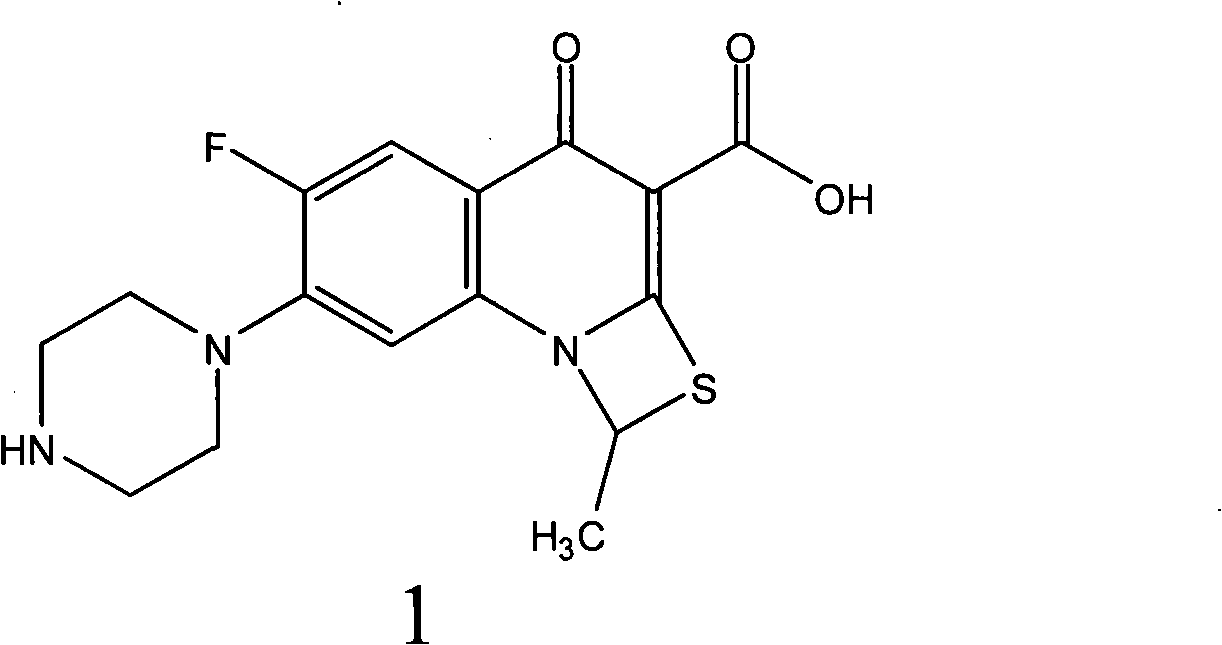
[0012] Dissolve 105 g of racemic ulifloxacin in 1500 mL of dimethyl sulfoxide, add dropwise a solution of 27 g of D-tartaric acid dissolved in 405 mL of dimethyl sulfoxide under stirring, turbidity and precipitation appear, and stir at room temperature for 20 hours , precipitated and filtered, and the resulting solid was dried under vacuum to obtain 86 grams. This solid was recrystallized in dimethyl sulfoxide to obtain 37 grams of L-ulifloxacin-D-tartrate. After elemental analysis, C49.08%, H5. 06%, N9.50%, S7.44% (molecular composition: C 16 h 16 FN 3 o 3 S·1 / 2C 4 h 6 o 6 ·H 2 O, calculated value C48.86%, H4.78, N9.50%, S7.25%); add this salt to water to form a suspension, adjust the pH value to 7-8 with 2% NaOH aqueous solution under stirring, precipitate and filter Dry to obtain (S)-ulifloxacin 24.5 grams, specific rotation (c=0.15, 0.1mol / LNaOH); 1 H-NMR (DMSO-d 6 )δ 2.11 (3H, d, j = 6.2Hz), 2.87 (4H, m)...
Embodiment 2
[0013] The preparation of embodiment 2 (R)-ulifloxacin
[0014] Dissolve 105 grams of racemic ulifloxacin in 1500 mL of DMSO, add dropwise a solution of 27 grams of L-tartaric acid dissolved in 405 mL of dimethyl sulfoxide under stirring, turbidity and precipitation appear, stir at room temperature for 20 hours, and filter to obtain The solid was dried under vacuum to obtain 82 grams, and the solid was recrystallized and purified in dimethyl sulfoxide to obtain 34 grams of dextro-ulifloxacin-L-tartrate. The salt was added to water to form a suspension, and stirred with 2% NaOH aqueous solution adjusted the pH value to 7-8, filtered and dried to obtain 22 grams of (R)-ulifloxacin, specific rotation (c=0.15, 0.1mol / LNaOH), optical purity e.e.>95%.
Embodiment 3
[0015] Embodiment 3 in vitro antibacterial test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com