Industrial compounding method of mule (benzo (e) (1,3) oxazine-2, 4'-piperidine)-4(3H)-ketonic
A synthetic method and the technology of piperidone, applied in the field of industrial synthesis of spiro[benzo[e][1,3]oxazine-2,4'-piperidin]4(3H)-one, to achieve easy reaction , low preparation cost, reasonable reaction process selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
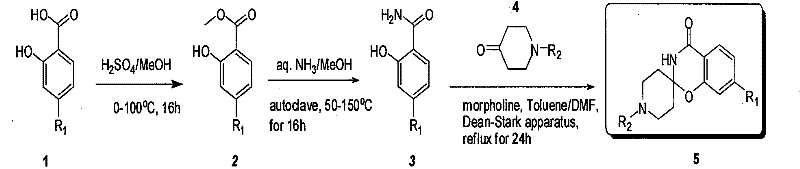
[0020] Add methyl 4-aminosalicylate (700g) to a 10L autoclave, add methanol (3.5L) and concentrated ammonia water (3.5L), seal and heat, stir at 100°C for 20 hours, cool down, and concentrate to dryness. Dichloromethane (3.5 L) was added, heated to reflux for 30 minutes, and cooled to room temperature. Filtration and washing of the filter cake with dichloromethane (200 mL) yielded 550 g of product. 1 H NMR (400MHz, DMSO-d 6 ): δ13.22(s, 1H, OH), 7.75(s, 1H, NH), 7.46(d, J=8.8Hz, 1H), 7.25(s, 1H, NH), 6.02(dd, J=8.4 Hz, 1H), 5.91(d, J=2.0 Hz, 1H), 5.71(s, 2H, NH)); Ms(M + +1, 153.1).
[0021] Synthesis of 7-aminospiro[benzo[e][1,3]-2,4'-Boc-piperidin]-4(3H)-one
[0022] 4-amino salicylamide (400 grams) joins in the there-necked flask of 10L, then adds N,N-dimethylformamide (1.3L), toluene (5L), N-Boc-4-piperidone ( 1050 g) and morpholine (230 g). Heated to 115°C, carried water through toluene, and heated to reflux for 20 hours. Cool down and concentrate to remove tolue...
Embodiment 2
[0027] 4-Amino salicylamide (30 grams) was added into a 2L there-necked flask, then N, N-dimethylformamide (200mL), toluene (400mL), p-toluenesulfonic acid (47.3 grams), N- Boc-4-piperidone (1050 g) and 4 Molecular sieves (10 g). Heated to 115°C, carried water through toluene, and heated to reflux for 48 hours. The temperature was lowered, and the pH was adjusted to 9 with ammonia methanol solution. Filter and concentrate the filtrate to dryness. Acetonitrile (200 mL) was added, filtered, and the filter cake was washed with acetonitrile to obtain 16 g of product with a yield of 32%. Its test data is as shown in above-mentioned embodiment 1.
[0028] Example 3
[0029] Synthesis of 4-aminomethyl salicylate
[0030] Add methanol (3L) to 4-aminosalicylic acid (300g), stir for 15 minutes, cool to 0-10°C, slowly add thionyl chloride (300g), return to room temperature, stir overnight, filter, and add acetic acid to the obtained solid Ethyl ester (3000mL) and water (3000m...
Embodiment 3
[0032] Add 4-aminosalicylic acid methyl ester (270g) to a 10L autoclave, add tetrahydrofuran (3L) and concentrated ammonia water (3L), seal and heat, stir at 100°C for 20 hours, cool down, and concentrate to dryness. Dichloromethane (3.5 L) was added, heated to reflux for 30 minutes, and cooled to room temperature. Filtration and washing of the filter cake with dichloromethane (200 mL) yielded 240 g of crude product. Its test data is as shown in above-mentioned embodiment 1.
[0033] Synthesis of 7-aminospiro[benzo[e][1,3]-2,4'-Boc-piperidin]-4(3H)-one
[0034] Add 4-amino salicylamide (200 grams) into a 10L three-necked flask, then add N,N-dimethylformamide (0.7L), toluene (2.5L), N-Boc-4-piperidone (500 grams) and piperidine (230 grams). Heated to 115°C, passed through toluene with water, and heated to reflux for 40 hours. Cool down and concentrate to remove toluene. Acetonitrile (2.5 L) was added, filtered, and the filter cake was washed with acetonitrile to obtain 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com