Method for regenerating catalyst for the production of methacrylic acid and process for preparing methacrylic acid
A methacrylic acid and catalyst technology, applied in chemical instruments and methods, catalyst regeneration/reactivation, chemical elements of heterogeneous catalysts, etc., can solve the problems of incomplete and satisfactory catalytic activity of regenerated catalysts, and achieve excellent selection Sexual, high-converting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
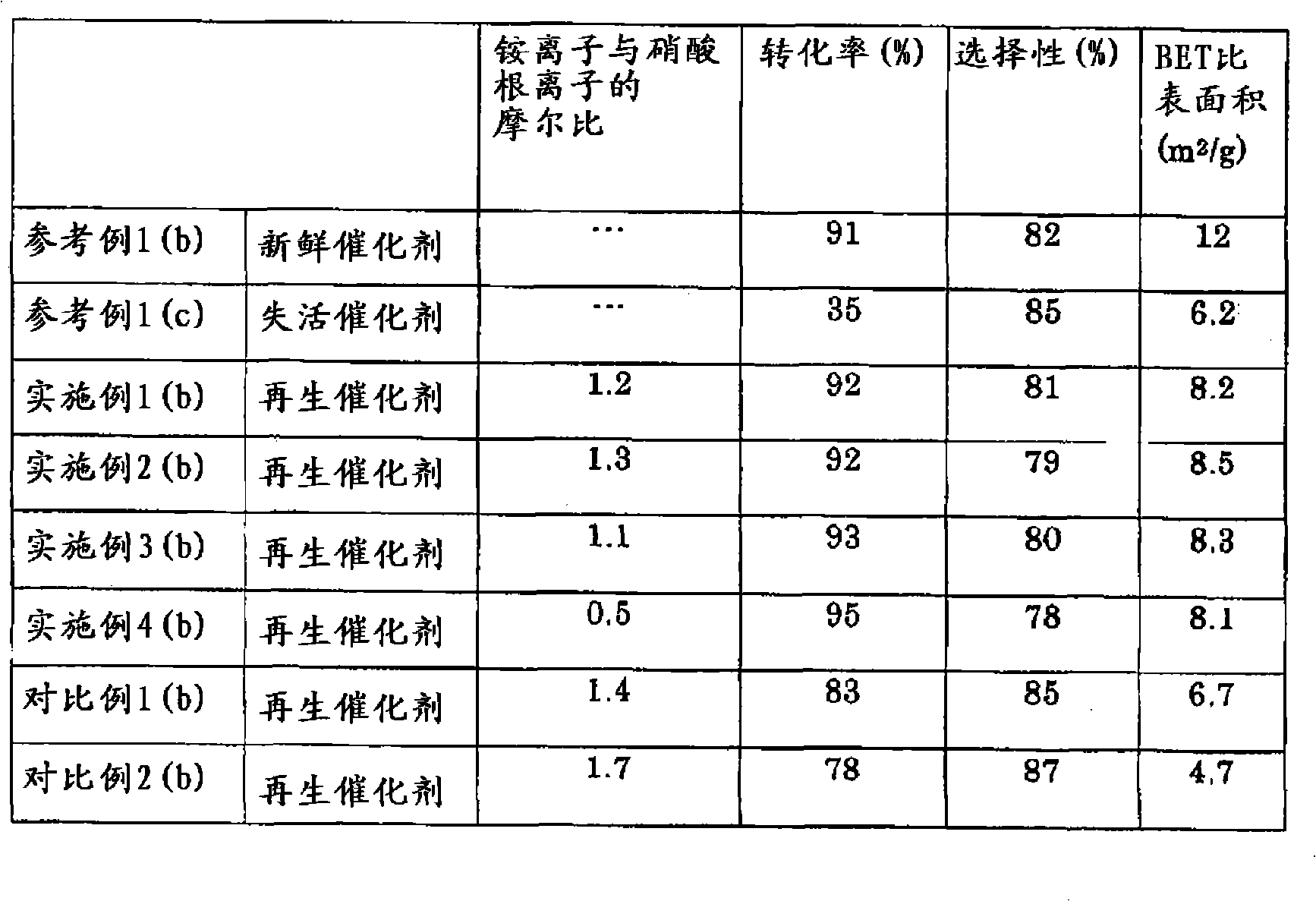
[0051] Preparation of Fresh Catalyst and Evaluation of Fresh Catalyst
[0052] 38.2kg cesium nitrate [CsNO 3 ], 27.4 kg of 75% by weight of orthophosphoric acid and 25.2 kg of 70% by weight of nitric acid were dissolved in 224 kg of ion-exchanged water heated to 40° C. to prepare solution A. Separately, 297kg ammonium molybdate tetrahydrate [(NH 4 ) 6 Mo 7 o 24 4H 2 O] was dissolved in 330kg of ion-exchanged water heated to 40°C, and then 8.19kg of ammonium metavanadate [NH 4 VO 3 ] suspended therein to prepare solution B. Solutions A and B were adjusted to 40 °C. After solution A was added dropwise to solution B while stirring, the mixture was further stirred in a closed container at a temperature of 120°C for 5.8 hours, and then 10.2 kg of antimony trioxide [Sb 2 o 3 ] and 10.2kg copper nitrate trihydrate [Cu(NO 3 ) 2 ·3H 2 O] Suspension in 23 kg deionized water. The mixture was then stirred at a temperature of 120° C. in a closed vessel for 5 hours. The mixtu...
Embodiment 1
[0061] Preparation of regenerated catalyst
[0062] Two hundred grams (200 g) of the deactivated catalyst obtained in Reference Example 1(c) was added to 400 g of ion-exchanged water and the mixture was stirred. The kinds and amounts of elements (consumed elements) missing from the deactivated catalyst compared with the fresh catalyst obtained in Reference Example 1(a) were determined from fluorescent X-ray analysis. Thus, 31.5 g of molybdenum trioxide (MoO 3 ) and 2.7 g of 75% by weight orthophosphoric acid to compensate for missing elements. Then add 69.2g ammonium nitrate (NH 4 NO 3 ), the mixture was heated to 70 °C and maintained at this temperature for 1 hour. Thereafter, 12.5 g of 25% by weight aqueous ammonia were added. After maintaining at a temperature of 70°C for 1 hour, the mixture was stirred at a temperature of 120°C for 5 hours in an airtight container. The molar ratio of ammonium ions to nitrate ions in the slurry is 1.2. The slurry was dried at a tempe...
Embodiment 2
[0067] Preparation of regenerated catalyst
[0068] A regenerated catalyst was obtained in the same manner as in Example 1(a), except that the amount of 25% by weight ammonia water was changed from 12.5 g to 16.9 g and the molar ratio of ammonium ions to nitrate ions was adjusted to 1.3. The BET specific surface area of the regenerated catalyst is shown in Table 1.
[0069] Example 2(b)
[0070] Activity test of regenerated catalyst
[0071] The regenerated catalyst obtained in Example 2(a) was tested for activity in the same manner as in Reference Example 1(b) to determine conversion and selectivity. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com