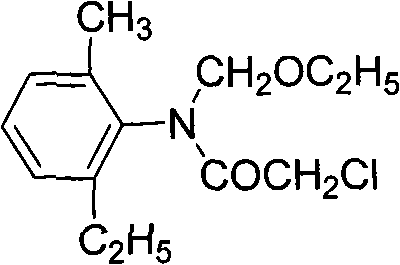
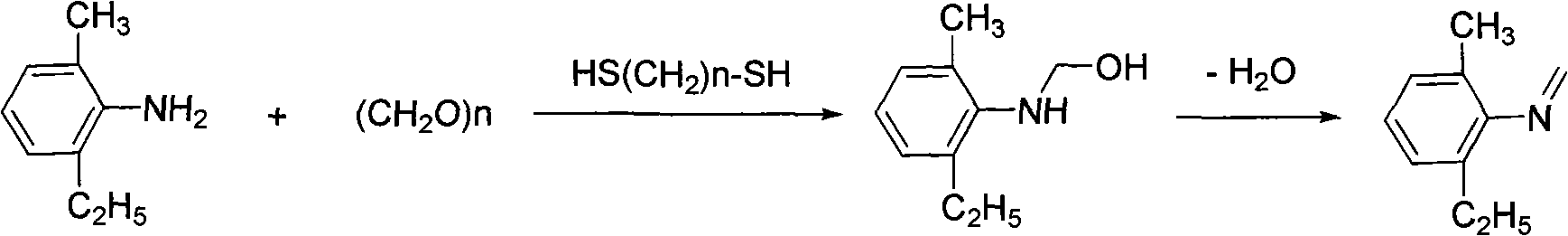Preparation method of N-methylene-2-methyl-6-ethylaniline
An ethylaniline and methylene technology, which is applied in the field of preparation of aniline compounds, can solve the problems of unstable methylidene, large environmental pollution, blocked pipelines, etc., and achieves improved synthesis yield and purity, excellent polymerization inhibition performance, The effect of stabilizing the reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 0.01:1
[0027] Take 151g of 99% 2-methyl-6-ethylaniline, 0.98g of ethylenedithiol with 99% polymerization inhibitor, 54.3g of paraformaldehyde, and 0.68g of triethylamine, and add them to a 500mL four-necked reaction flask , stirred, heated to 100° C., reacted for 2 hours, dehydrated under reduced pressure, and then evaporated excess formaldehyde under reduced pressure to obtain 160.1 g of methylene, with a content of 98.0% and a yield of 96.39%.
Embodiment 2
[0028] Example 2 0.02:1
[0029] Take 151g of 99% 2-methyl-6-ethylaniline, 1.95g of ethylenedithiol with 99% polymerization inhibitor, 54.3g of paraformaldehyde, and 0.68g of triethylamine, and add them to a 500mL four-necked reaction flask , stirred, heated to 95° C., reacted for 2 hours, dehydrated under reduced pressure, and then evaporated excess formaldehyde under reduced pressure to obtain 161.7 g of methylene, with a content of 98.5% and a yield of 97.85%.
[0030] Take 136.7g of 98.5% chloroacetyl chloride and put it into a 500ml four-necked reaction flask. After nitrogen replacement, add 161.7g of 98.5% methylene chloride, and keep the reaction at 80-100°C for 2 hours.
[0031] Add 75g of absolute ethanol to another 500ml four-necked bottle, lower the temperature to 0-10°C, then transfer the above-mentioned reaction materials to the dropping funnel while hot, and complete the dropwise addition within 1 hour, 50-55 The reaction was incubated between ℃ for 2 hours. Af...
Embodiment 3
[0032] Example 3 0.04:1
[0033] Take 151g of 99% 2-methyl-6-ethylaniline, 3.9g of ethylenedithiol with 99% polymerization inhibitor, 54.3g of paraformaldehyde, and 0.68g of triethylamine, and add them to a 500mL four-necked reaction flask , stirred, heated to 85° C., reacted for 2 hours, dehydrated under reduced pressure, and then evaporated excess formaldehyde under reduced pressure to obtain 162 g of methylene, with a content of 98.4% and a yield of 97.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com