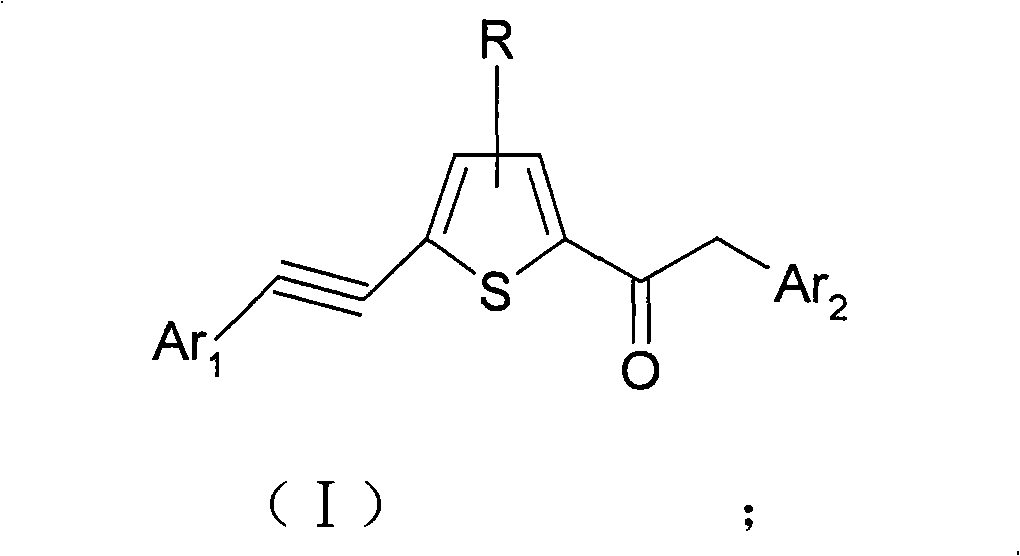
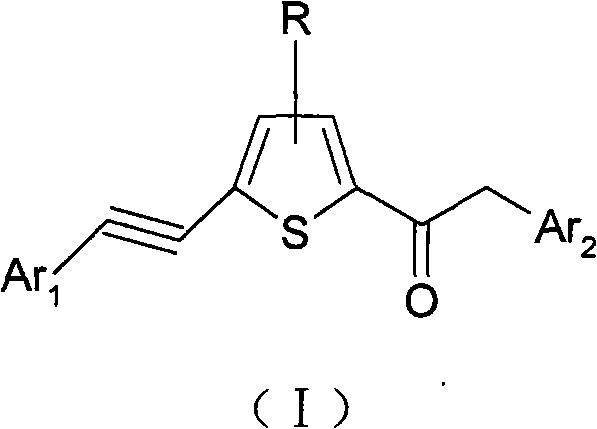
Alkynyl thiophene ketone compound and preparation method and applications thereof
A technology of alkynyl thiophenone and compound, applied in the field of chemistry, can solve problems such as high synthesis cost and few types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1: Preparation of 2-phenyl-1-(5-(2-phenylethynyl)thiophen-2-yl)ethanone
[0127] Weigh 18.4g (0.1mol) of 2-(2-phenylethynyl)thiophene and add it to 50ml of dichloromethane, add 2g of phosphoric acid as a catalyst, and add 15.4g of 2-phenylacetyl chloride to 50ml of dichloromethane dropwise at room temperature solution, stirring while dripping. After the dropwise addition was completed, the stirring reaction was continued for 2 hours, and the thin layer indicated the end point of the reaction. The product (compound 1) was obtained by water washing, extraction, solvent removal, and ethanol recrystallization according to routine laboratory methods.
Embodiment 2
[0128] Example 2: Preparation of 2-(naphthalene-2-yl)-1-(5-(2-phenylethynyl)thiophen-2-yl)ethanone
[0129] Weigh 18.4g (0.1mol) of 2-(2-phenylethynyl)thiophene and add it to 50ml of dichloromethane, add 2g of phosphoric acid as a catalyst, and add 20.4g of 2-(naphthyl-2-yl)ethane dropwise at room temperature Acyl chloride in 50ml of dichloromethane solution, stirring while dropping. Continue to stir the reaction after the dropwise addition, and the thin layer indicates the end point of the reaction. The product (compound 2) was obtained by washing with water, extracting, removing the solvent, and recrystallizing with ethanol according to routine laboratory methods.
Embodiment 3
[0130] Example 3: Preparation of 2-(thiophen-2-yl)-1-(5-(2-(thiophen-2-yl)ethynyl)thiophen-2-yl)ethanone
[0131] Weigh 18.9g (0.1mol) of 2-(2-(thiophen-2-yl)ethynyl)thiophene and add it to 50ml of dichloromethane, add 2g of phosphoric acid as a catalyst, and add 15.9g of 2-(thiophene-2 -base) 50ml dichloromethane solution of acetyl chloride, stirring while dropping. Continue to stir the reaction after the dropwise addition, and the thin layer indicates the end point of the reaction. The product (compound 4) was obtained by washing with water, extracting, removing the solvent, and recrystallizing with ethanol according to routine laboratory methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com