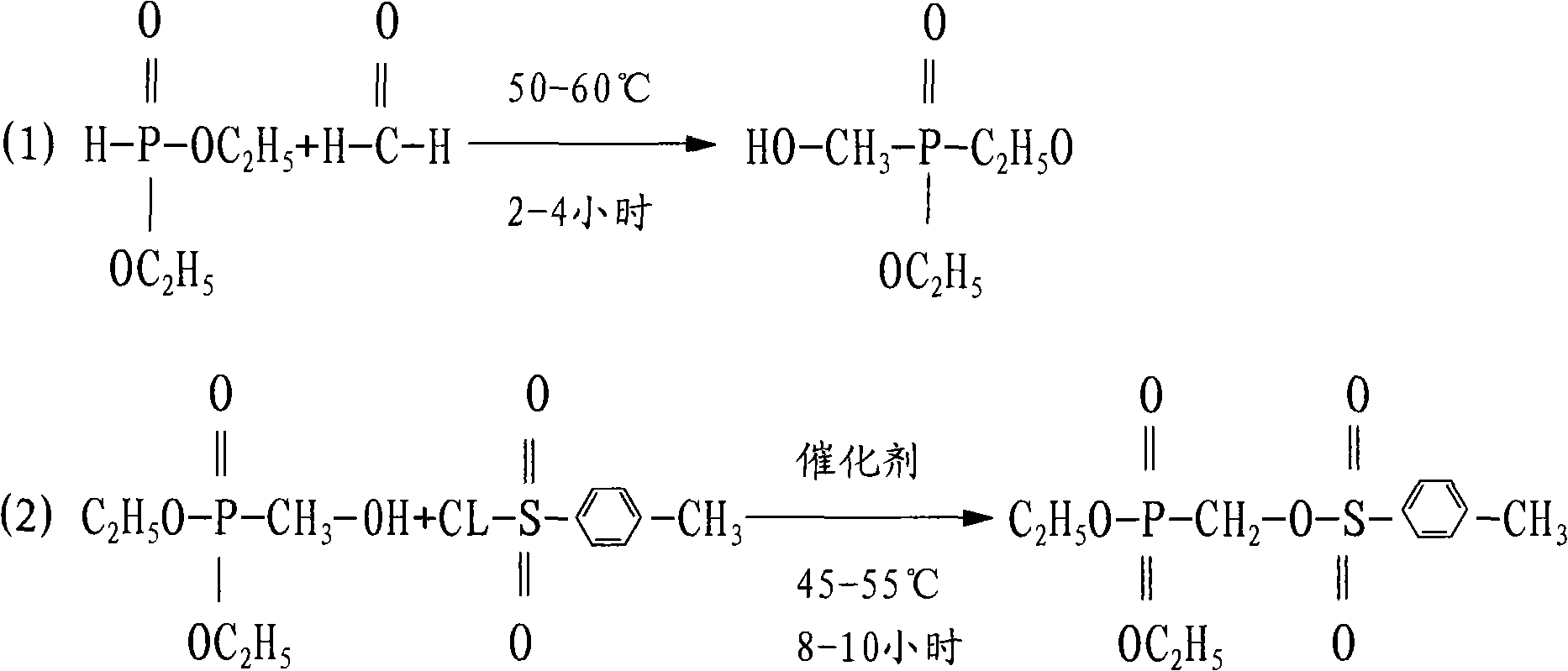New production technology of diethyl (tosyloxy)methylphosphonate
A technology of diethyl toluenesulfonyloxymethylphosphonate and diethyl hydroxymethylphosphonate is applied in the field of new technology for producing diethyl p-toluenesulfonyloxymethylphosphonate, which can solve the problem of insufficient reaction, insufficient reaction, The problems of increased environmental pollution and long reaction time have achieved the effects of low cost, reduced pollution and extended service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
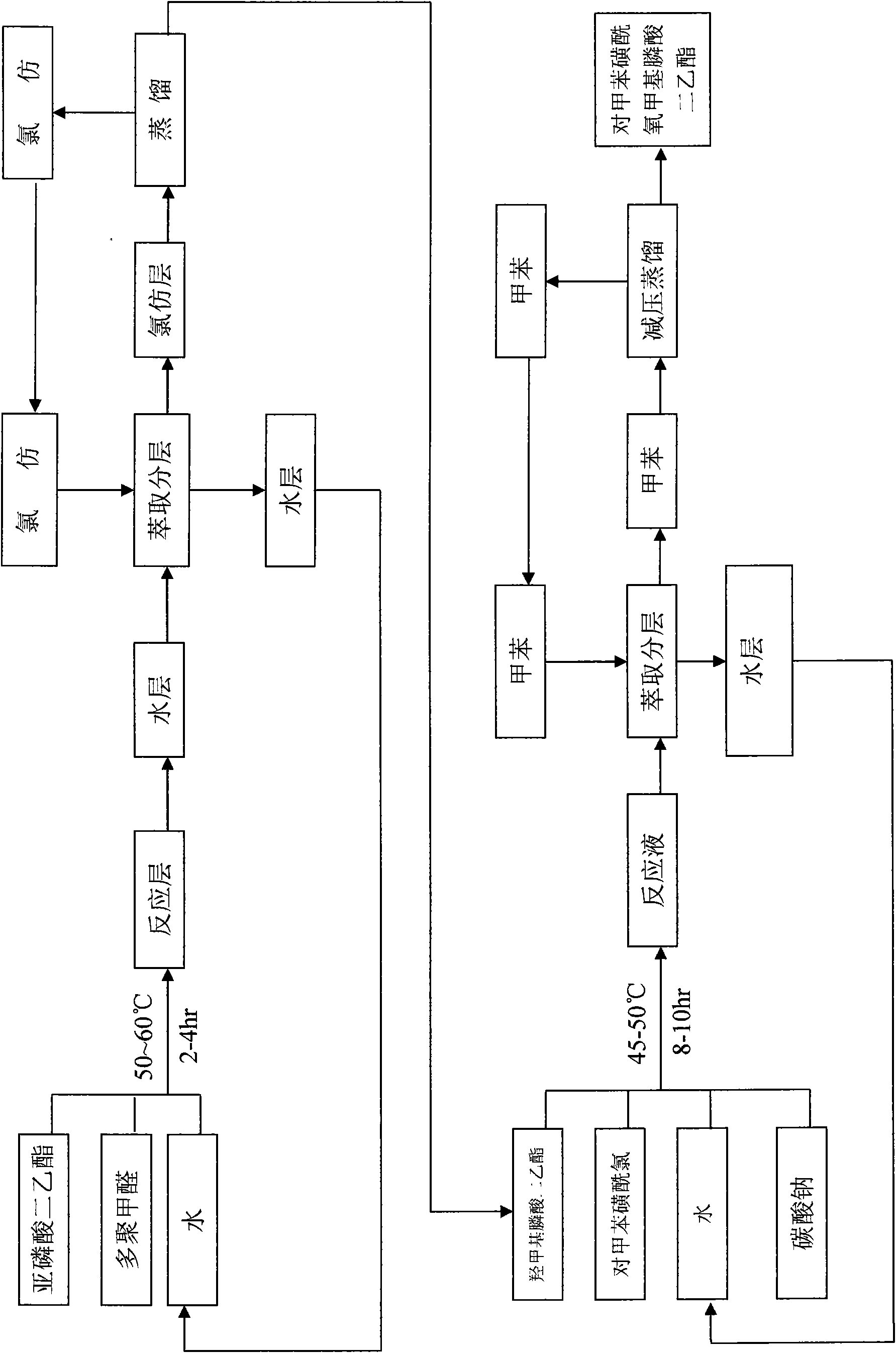
[0019] Example 1: (1) Synthesis of diethyl hydroxymethylphosphonate Take 70kg of diethyl phosphite, 10kg of paraformaldehyde, and 30kg of water respectively in the reactor, start stirring, and heat up to between 50-60°C , heat preservation reaction for 2-4 hours, after the reaction is completed, drop to room temperature, continue to add 150kg of chloroform for extraction, separate the layers, separate the water layer and recycle it, and distill the organic layer under normal pressure to recycle the chloroform to obtain diethyl hydroxymethylphosphonate as the distillation residue 85kg.
[0020] (2) Synthesis of diethyl p-toluenesulfonyloxymethylphosphonate Get 50kg water, 150kg p-toluenesulfonyl chloride, 70kg diethyl hydroxymethylphosphonate, 50kg ammonia water, heat up to 45-55°C for 8-10 hour, down to room temperature, add 300kg toluene for extraction, separate the water layer and reclaim it for mechanical use. After the toluene layer is filtered, reclaim the toluene under r...
Embodiment 2
[0021] Example 2: (1) Synthesis of diethyl hydroxymethyl phosphonate Take 100kg of diethyl phosphite, 30kg of paraformaldehyde, and 50kg of water respectively in the reactor, start stirring, and heat up to between 50-60°C , heat preservation reaction for 2-4 hours, after the reaction is completed, it is lowered to room temperature, continue to add 150kg of chloroform for extraction, separate layers, separate the water layer and recover it for mechanical use, distill the organic layer at normal pressure to recover chloroform, and obtain the distillation residue diethyl hydroxymethylphosphonic acid Ester 118kg.
[0022] (2) Synthesis of diethyl p-toluenesulfonyloxymethylphosphonate Get 120kg water, 230kg p-toluenesulfonyl chloride, 100kg diethyl hydroxymethylphosphonate, 100kg ammonia water, heat up to 45-55°C for 8-10 hour, down to room temperature, add 350kg of toluene for extraction, separate the water layer and reclaim for use mechanically, and reclaim toluene under reduced ...
Embodiment 3
[0023] Embodiment 3: (1) Synthesis of diethyl hydroxymethyl phosphate Get 85kg of diethyl phosphite, 20kg of paraformaldehyde, and 40kg of water respectively in the reactor, start stirring, and heat up to between 50-60°C. Insulate and react for 2-4 hours, after the reaction is completed, cool down to room temperature, continue to add 150kg of chloroform for extraction, separate layers, separate the water layer and recover it for mechanical use, distill the organic layer under normal pressure to recover chloroform, and obtain 105kg of diethyl hydroxymethylphosphonate as the distillation residue .
[0024] (2) Synthesis of diethyl p-toluenesulfonyloxymethylphosphonate Get 80kg water, 180kg p-toluenesulfonyl chloride, 85kg diethyl hydroxymethylphosphonate, 80kg ammonia water, heat up to 45-55°C for 8-10 hour, down to room temperature, add 320kg of toluene for extraction, separate the water layer and reclaim for mechanical use, and reclaim toluene under reduced pressure after the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com