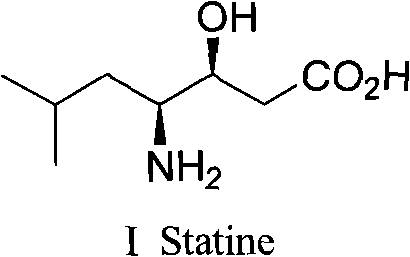
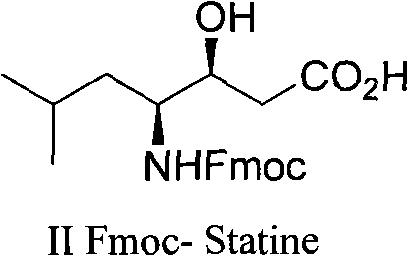
Preparation method of (3S, 4S)-4-amino-3-hydroxy-6-methylheptanoic acid and analogue thereof
A technology of methylheptanoic acid and its analogs, which is applied in the field of preparation of amino acid compounds, can solve problems such as unfavorable large-scale production, and achieve the effects of correct structure, easy operation and amplification, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
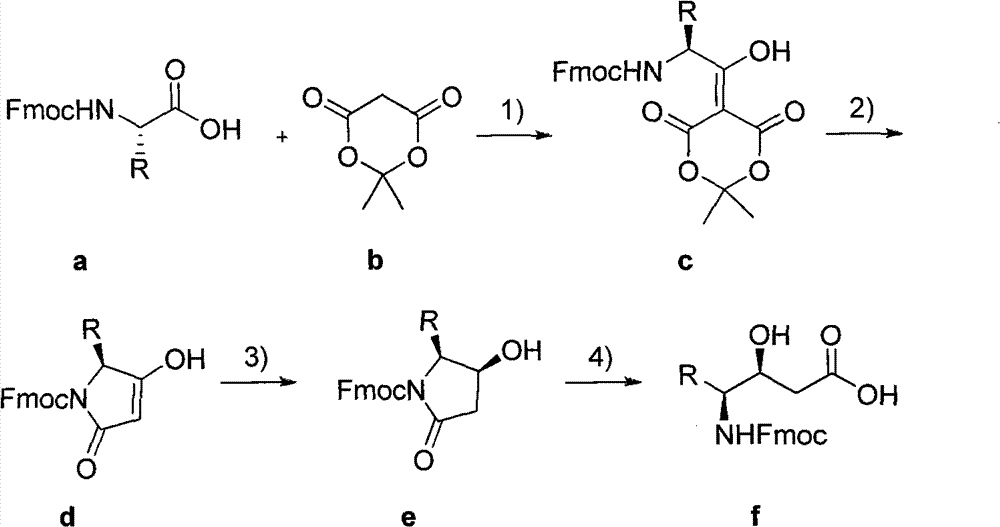
[0027] The synthesis of embodiment 1 Fmoc-Statine and Statine (R=(CH 3 ) 2 CHCH 2 -isopropylmethylene)
[0028]Add 884 mg (2.5 mmol) of amino acid (a) (R is isopropyl methylene) protected by Fmoc at the nitrogen end, 10 mL of dichloromethane, 360 mg (2.5 mmol) of Meldrum's acid (b) and DMAP ( 4-Dimethylaminopyridine) 452 mg (3.7 mmol). After stirring and dissolving, slowly drop DIC 0.47mL (3.0mmol), react at room temperature for 3.5h, pour into 120mL 0°C cold ethyl acetate, and use cold 5% NaHSO 4 Aqueous solution (3×30 mL), cold water (3×30 mL) and washed with saturated brine 30 mL. Ethyl acetate organic layer with anhydrous MgSO 4 Dry for 6h. After filtration, the filtrate was concentrated to about 30 mL by rotary evaporation, heated to reflux in ethyl acetate on a water bath for 1 h, and the solvent was distilled off under reduced pressure to obtain a crude product of light red gum d.
[0029] Then add 10 mL of dichloromethane and 1 mL of acetic acid, and slowly add ...
Embodiment 2
[0032] The synthesis of embodiment 2 Fmoc-Statine (R=(CH 3 ) 2 CHCH 2 -isopropylmethylene)
[0033] 22.1 g (62.5 mmol) of a, 250 mL of dichloromethane, 9.0 g (62.5 mmol) of b and 13.0 g (106.3 mmol) of DMAP were added to a round bottom flask. After stirring and dissolving, slowly drop DIC 13.6mL (87.5mmol), react at room temperature for 3.5h, pour into 1500mL 4°C cold ethyl acetate, and use cold 5% NaHSO 4 Aqueous solution (3×500 mL), cold water (3×500 mL) and washed with saturated brine 250 mL. Anhydrous MgSO for organic layer 4 Dry for 6h. After filtration, the filtrate was concentrated to about 500 mL by rotary evaporation, heated to reflux in ethyl acetate on a water bath for 3 h, and the solvent was evaporated under reduced pressure to obtain a crude product of light red gum d.
[0034] Then add 250 mL of dichloromethane and 20 mL of acetic acid, and slowly add NaBH under cooling in an ice-water bath at 4°C. 4 3.6g (93.8mmol), kept stirring at 0°C for 3.5h. The re...
Embodiment 3
[0036] Example 3 Synthesis of Fmoc-Statine analogs and Statine analogs (R=Benzyl benzyl)
[0037] Add a (R is benzyl) 9.69g (25mmol), dichloromethane 100mL, b 3.6mg (25mmol) and DMAP5.5g (45mmol) in a round bottom flask. After stirring and dissolving, slowly drop DIC 4.3mL (27.5mmol), react at room temperature for 3.5h, pour into 1200mL 4°C cold ethyl acetate, and use cold 5% NaHSO 4 Aqueous solution (3×300 mL), cold water (3×300 mL) and washed with saturated brine 300 mL. Anhydrous MgSO for organic layer 4 Dry for 6h. After filtration, the filtrate was concentrated to about 200 mL by rotary evaporation, heated to reflux in ethyl acetate on a water bath for 1 h, and the solvent was distilled off under reduced pressure to obtain a crude product of light red gum d.
[0038] Then add 100 mL of dichloromethane and 11.4 mL of acetic acid, and slowly add NaBH under cooling in an ice-water bath at 0°C. 4 1.9g (50mmol), kept stirring at 0°C for 3.5h. The reaction solution was pou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com