Method for preparing amorphous calcium carbonate by microemulsion method
A technology of amorphous calcium carbonate and microemulsion method, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problems of amorphous calcium carbonate purity to be improved, poor controllability of the preparation process, etc., and achieve favorable control and expansion of the reaction Reaction time range, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] CaCl 2 2H 2 O powder and dimethyl carbonate were placed in a molar ratio of 1:1 containing CaCl 2 2H 2 In a beaker of distilled water with a molar ratio of O of 2778:1, it was dispersed evenly at a stirring speed of 500 rpm to form a microemulsion. will be compared with CaCl 2 2H 2 Na with O molar ratio of 1:1 3 C 6 h 5 o 7 2H 2 Add the O powder to the above liquid and dissolve completely under stirring. will be compared with CaCl 2 2H 2 O NaOH powder with a molar ratio of 2:1 was added to the above mixture, and the stirring was continued for 2 minutes, and the aging time was 3 hours. Finally, the turbid liquid was suction-filtered and dried at 25°C to collect the white product.
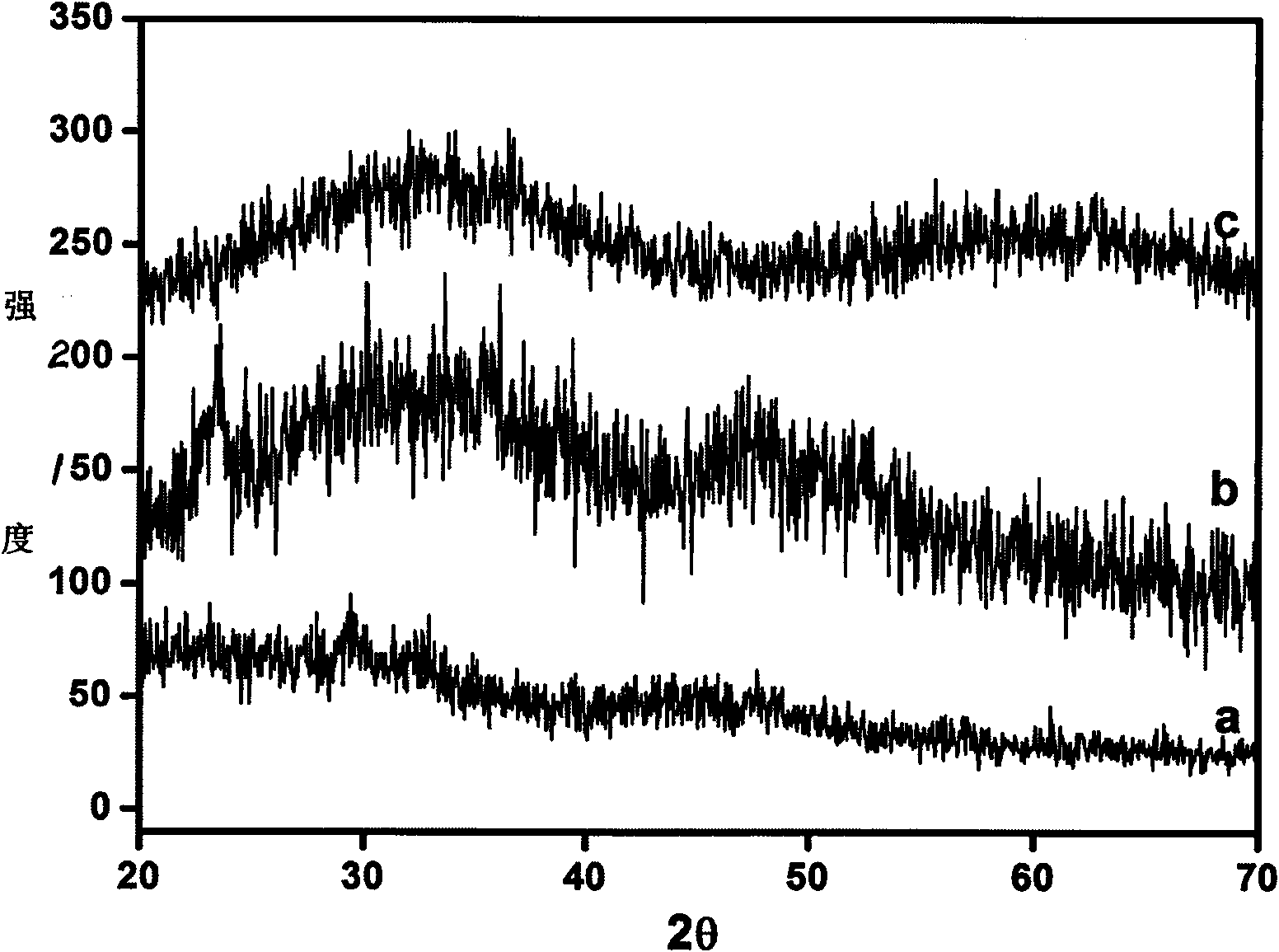
[0027] The obtained product was characterized by X-ray diffractometer (XRD), field emission scanning electron microscope (SEM) and field emission transmission electron microscope (TEM). The XRD pattern of the product is shown in figure 1 , it is clearly seen from the spectrum th...
Embodiment 2
[0029] CaCl 2 2H 2 O powder and dimethyl carbonate were placed in a molar ratio of 1:2 containing CaCl 2 2H 2 In a beaker of distilled water with a molar ratio of O of 5556:1, disperse it evenly at a stirring speed of 1000 rpm to form a microemulsion. will be compared with CaCl 2 2H 2 Add sodium aconitate with a molar ratio of 2:1 to the above liquid, and dissolve completely under stirring. will be compared with CaCl 2 2H 2 O NaOH powder with a molar ratio of 2:1 was added to the above mixture, and the stirring was continued for 4 minutes, and the aging time was 6 hours. Finally, the turbid liquid was suction-filtered and dried at 27°C to collect the white product.
[0030] The obtained product was characterized by X-ray diffractometer (XRD), field emission scanning electron microscope (FESEM) and transmission electron microscope (TEM). The XRD pattern of the product is shown in figure 1 , it is clear from the spectrogram that the product is amorphous calcium carbona...
Embodiment 3
[0032] CaCl 2 2H 2 O powder and dimethyl carbonate were placed in a molar ratio of 1:3 containing CaCl 2 2H 2 In a beaker of distilled water with a molar ratio of O of 8334:1, disperse it evenly at a stirring speed of 300 rpm to form a microemulsion. will be compared with CaCl 2 2H 2 Add the sodium trimesisate with a molar ratio of 1:2 to the above liquid, and dissolve completely under stirring. will be compared with CaCl 2 2H 2O NaOH powder with a molar ratio of 2:1 was added to the above mixed solution, stirring was continued for 1 min, and the aging time was 9 h. Finally, the turbid liquid was suction-filtered, dried at 20°C, and the white product was collected.
[0033] The obtained product was characterized by X-ray diffractometer (XRD), field emission scanning electron microscope (FESEM) and transmission electron microscope (TEM). The XRD pattern of the product is shown in figure 1 , it is clearly seen from the spectrum that the product is an amorphous calcium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com