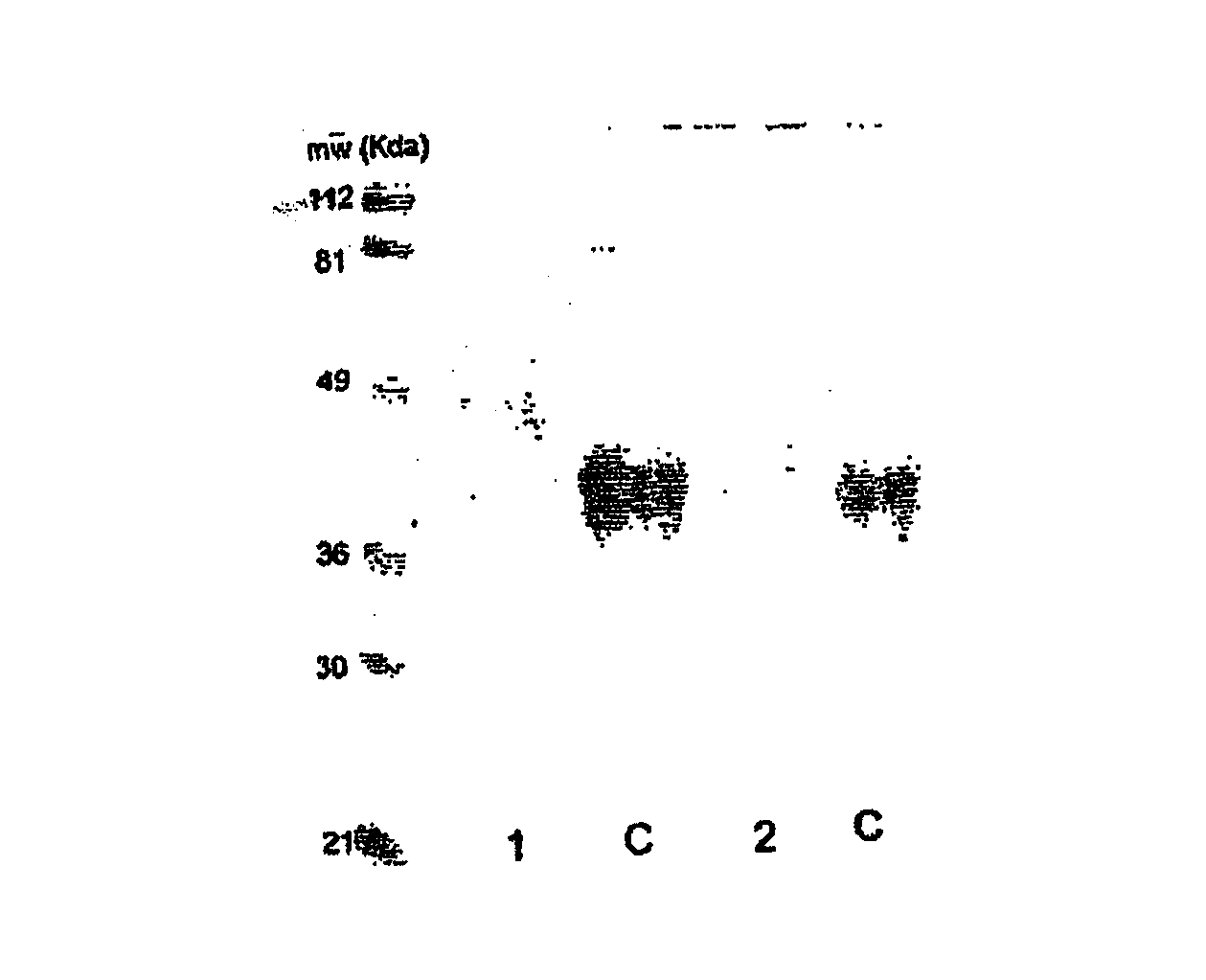
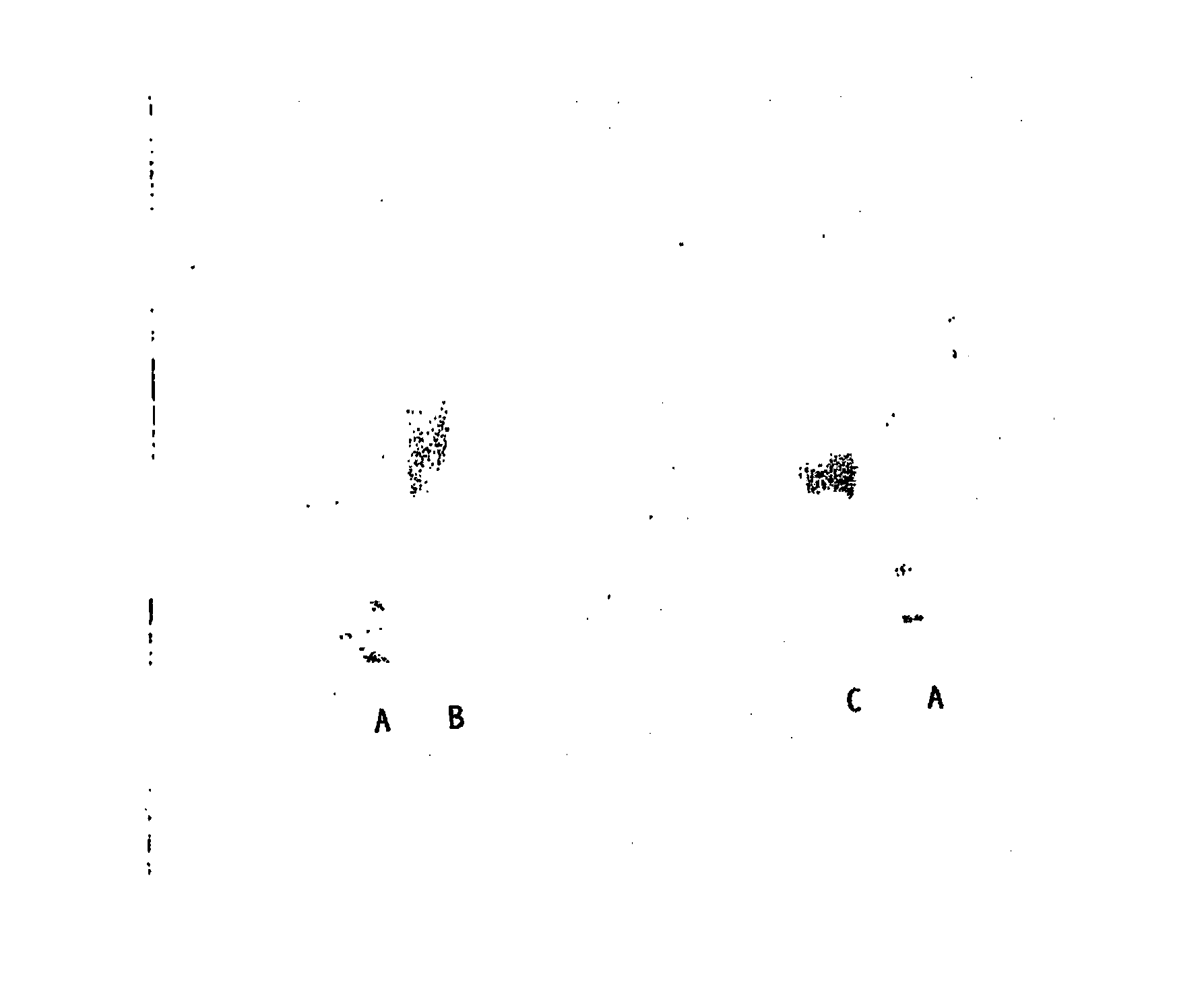Hasylated polypeptides, especially hasylated erythropoietin
A technology of erythropoietin and polypeptide, which is applied in the direction of erythropoietin, interleukin, cytokines/lymphokines/interferon, etc. It can solve the problems of lack of glycosylation, difficulty in producing polypeptides, and polypeptides not having correct folding and natural conformation etc. to achieve high biological activity and improved biological stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0517] Production of recombinant EPO
[0518] A) Production in mammalian cells
[0519] Recombinant EPO was produced in CHO cells as follows
[0520] A plasmid carrying human EPO cDNA was cloned into a eukaryotic expression vector (pCR3, hereinafter referred to as pCREPO). Site-directed mutagenesis was performed using standard methods as described (Grabenhorst, Nimtz, Costa et al., 1998, In vivo specificity of human alpha1, 3 / 4-fucosyltransferases III-VII in the biosynthesis of Lewis(x) and sialyl Lewis(x) motifs on complex-type N-glycans-Coexpression studies from BHK-21 cells together with humanbeta-trace protein, J. Biol. Chem., 273(47), 30985-30994).
[0521] CHO cells stably expressing human EPO or its amino acid variants (e.g., Cys-29→Ser / Ala, or Cys-33→Ser / Ala, Ser-126→Ala, etc.) were generated by calcium phosphate precipitation as described (Grabenhorst et al. Human) was screened with sulfate G418. Three days after transfection, the cells were subcultured 1:5 and ...
Embodiment 2
[0533] Formation of reactive HES derivatives
[0534] 1. SH-reactive HES
[0535] 1.1 Reaction of EMCH with oxo-HES12KD to form SH-reactive HES 12KD B
[0536]
[0537] Dissolve 0.144g (0.012mmol) of oxo-HES12KD (Fresenius German Patent DE 196 28 705 A1) in 0.3mL of anhydrous dimethylsulfoxide (DMSO) and add dropwise to 34mg (0.15mmol) of EMCH (Perbio Science, Deutschland GmbH, Bonn, Germany) in 1.5 mL of DMSO. After stirring at 60 for 19 hours, the reaction mixture was added to 16 mL of a 1:1 mixture of ethanol and acetone. The precipitate was collected by centrifugation, redissolved in 3 mL DMSO, and reprecipitated as above. SH-reactive HES 12KDB was obtained by centrifugation and vacuum drying. The coupling reaction with thiol-EPO is described in Example 3, 2.2.
[0538] Options:
[0539] All crosslinkers with hydrazide and maleimide functionalities separated by a spacer can be used in this reaction. Table 2 lists some further examples of such molecules availab...
Embodiment 3
[0603] Coupling reaction with thiol-EPO
[0604] 1. Reaction of mercapto-EPO with haloacetamide-modified SH-reactive HES
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com