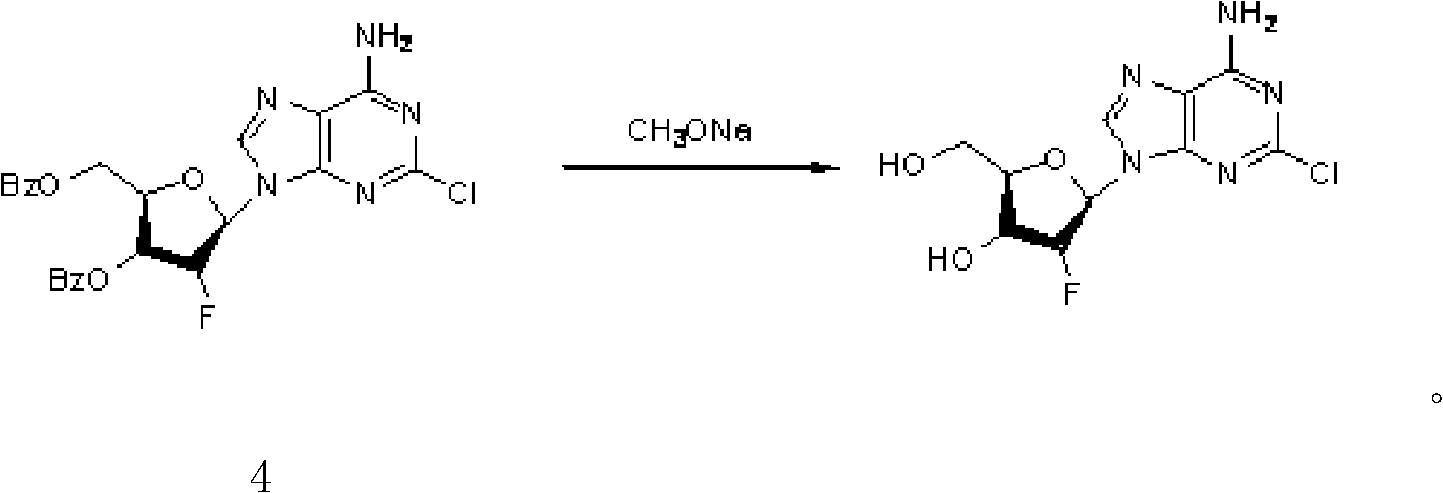
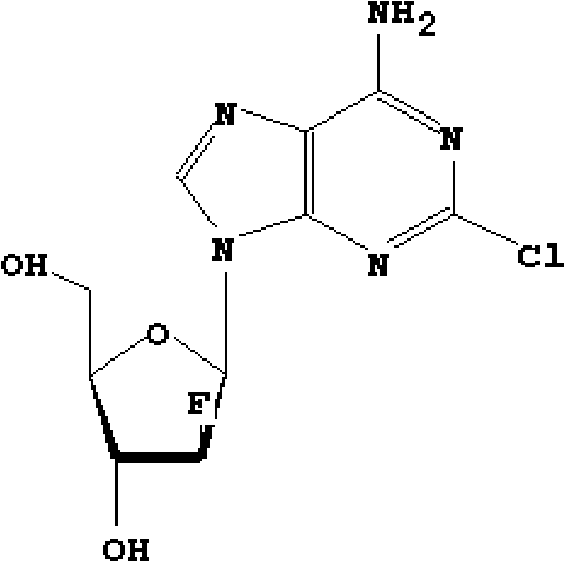
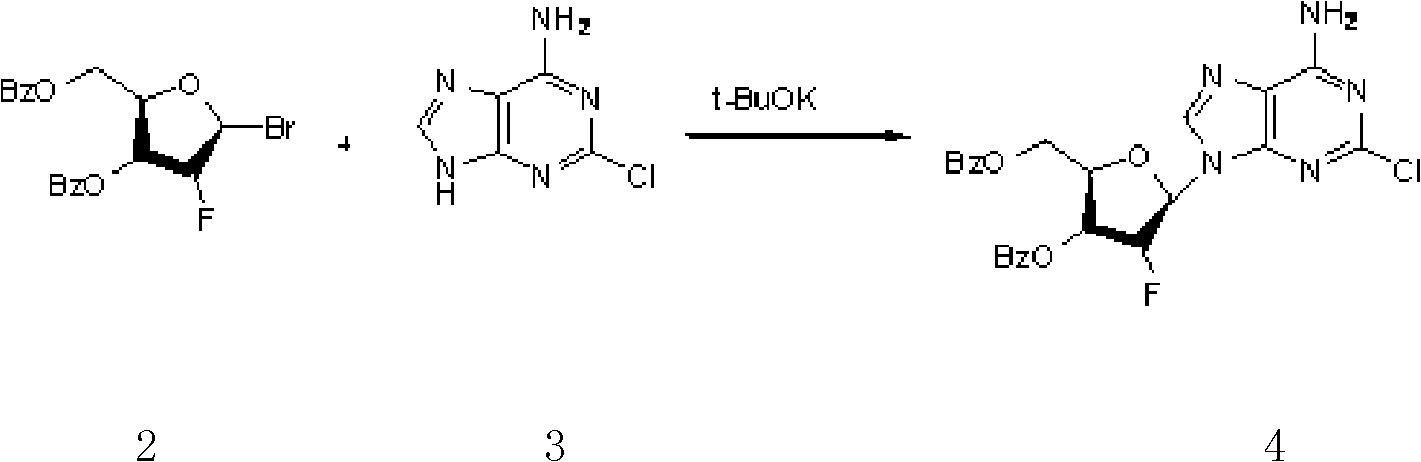Clofarabine pharmaceutical composition freeze-dried powder injection and preparation method thereof
A technology of freeze-dried powder injection and clofarabine, which is applied in the field of drug synthesis and preparation, can solve the problems of poor stability, unsuitable storage, and low solubility of freeze-dried products, and achieve good resolubility and clarity, and product reconstitution. Good solubility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] This embodiment relates to clofarabine pharmaceutical composition freeze-dried powder injection and its preparation method.
[0090] 1. Prescription of clofarabine pharmaceutical composition freeze-dried powder injection
[0091] Specification: 20mg / bottle
[0092] Raw material name dosage
[0093] Clofarabine (dried and pure) 20g
[0094] Mannitol 200g
[0095] Sodium bicarbonate 40g
[0096] Hydrochloric acid amount
[0097] Add water for injection to 10000ml
[0098] Makes 1000 bottles
[0099] 2. Preparation method
[0100] (1) take respectively the clofarabine and sodium bicarbonate of recipe quantity, mix it homogeneously, obtain the solid mixture of clofarabine and sodium bicarbonate;
[0101] (2) Add 80% water for injection of the prescription amount to the solid mixture obtained in step (1), heat while stirring, so that the temperature of the solution rises to 60° C., keep stirring to dissolve the solid mixture; add the manna of the prescription amount ...
Embodiment 2
[0108] This embodiment relates to the prescription and preparation method of clofarabine pharmaceutical composition freeze-dried powder injection
[0109] 1. Prescription of clofarabine pharmaceutical composition freeze-dried powder injection
[0110] Specification: 10mg / bottle
[0111] Raw material name Feed amount
[0112] Clofarabine (dried and pure) 10g
[0113] Mannitol 200g
[0114] Sodium bicarbonate 40g
[0115] Hydrochloric acid amount
[0116] Add water for injection to 10000ml
[0117] Makes 1000 bottles
[0118] 2. Preparation method
[0119] (1) take respectively the clofarabine and sodium bicarbonate of recipe quantity, mix it homogeneously, obtain the solid mixture of clofarabine and sodium bicarbonate;
[0120] (2) Add 70% water for injection of the prescription amount to the solid mixture obtained in step (1), heat while stirring, so that the temperature of the solution rises to 70° C., keep stirring to dissolve the solid mixture; add the manna of the ...
Embodiment 3
[0127] This embodiment relates to the prescription and preparation method of clofarabine pharmaceutical composition freeze-dried powder injection
[0128] 1. Prescription of clofarabine pharmaceutical composition freeze-dried powder injection
[0129] Specification: 30mg / bottle
[0130] Raw material name Feed amount
[0131] Clofarabine (dried and pure) 30g
[0132] Mannitol 300g
[0133] Sodium bicarbonate 60g
[0134] Hydrochloric acid amount
[0135] Add water for injection to 10000ml
[0136] Makes 1000 bottles
[0137] 2. Preparation method
[0138] (1) take respectively the clofarabine and sodium bicarbonate of recipe quantity, mix it homogeneously, obtain the solid mixture of clofarabine and sodium bicarbonate;
[0139](2) Add 80% water for injection of the prescription amount to the solid mixture obtained in step (1), heat while stirring, so that the temperature of the solution rises to 80° C., keep stirring to dissolve the solid mixture; add the manna of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com