Clopidogrel hydrogen sulfate tablet and preparation method thereof
A clopidogrel bisulfate tablet and a technology of clopidogrel bisulfate, which are applied in the field of medicine, can solve problems such as lack of anti-platelet aggregation, and achieve the effect of improving safety, effectiveness and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
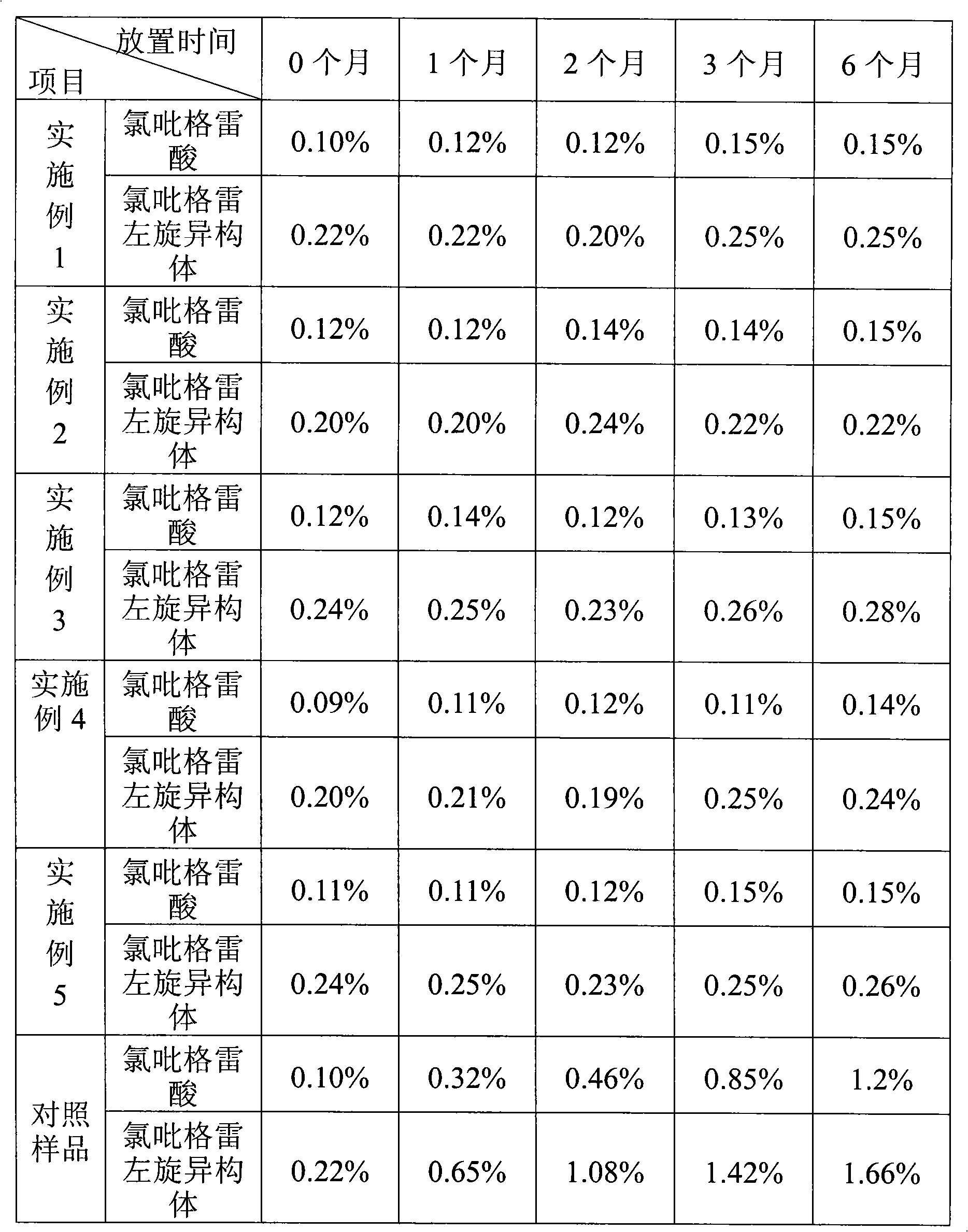
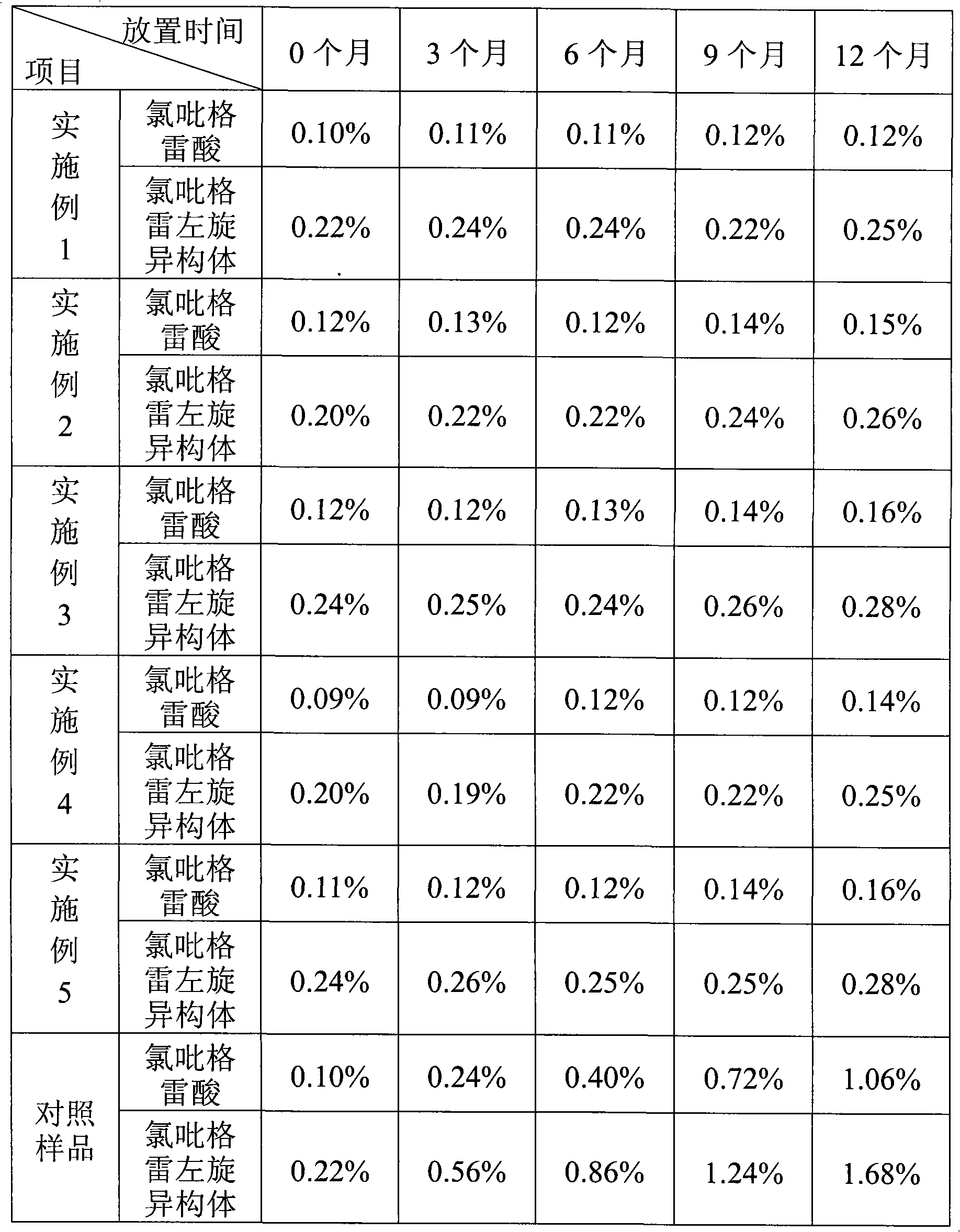
Embodiment 1
[0010] Embodiment 1 prescription: (make 1000)
[0011] Main drug
[0012] Preparation method: mix vitamin C with microcrystalline cellulose according to the method of equal amount addition, and then mix with the main drug, mannitol, polyethylene glycol 4000, part of croscarmellose sodium, part of micro-powdered silica gel, and part of talcum powder Finally, the granules are prepared by dry granulation, and the obtained granules are mixed evenly with the remaining croscarmellose sodium, micronized silica gel, and talcum powder, pressed into tablets, coated with film, and packaged.
Embodiment 2
[0013] Embodiment 2 prescription: (make 1000)
[0014] Main drug
[0015] Preparation method: Mix vitamin C with microcrystalline cellulose according to the method of equal amount addition, and then granulate with the main drug, lactose, pregelatinized starch, polyethylene glycol 6000, and part of carboxymethyl starch sodium, and use water as the wetting agent. Drying, granulation, adding the remaining sodium starch glycolate, micropowder silica gel, and talcum powder, mixing evenly, pressing into tablets, coating with film, and packaging.
Embodiment 3
[0017] Prescription: (makes 1000 tablets)
[0018] Main drug
[0019] Preparation method: Dissolve vitamin C in a certain amount of water, add 95% ethanol solution to make an ethanol solution containing 50% alcohol, and obtain a wetting agent. After mixing mannitol and microcrystalline cellulose, completely wet it with a wetting agent, and dry it to obtain material 1. Take the main drug, material 1, pregelatinized starch, polyethylene glycol 6000, part of cross-linked povidone, part of micropowder silica gel, part of talc powder and mix them to make granules by dry granulation method. Vitone, micropowder silica gel, and talcum powder are mixed uniformly, pressed into tablets, and coated with a film.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com