Method for preparing chlorfenapyr and analog thereof
An analog, the technology of chlorfenapyr, which is applied in the field of preparation of chlorfenapyr and its analogs, can solve the problems that triethylamine cannot be directly applied mechanically, the acid binding agent is expensive, the recovery rate is not high, etc., and achieves good industrial application prospects, The effect of low production cost and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
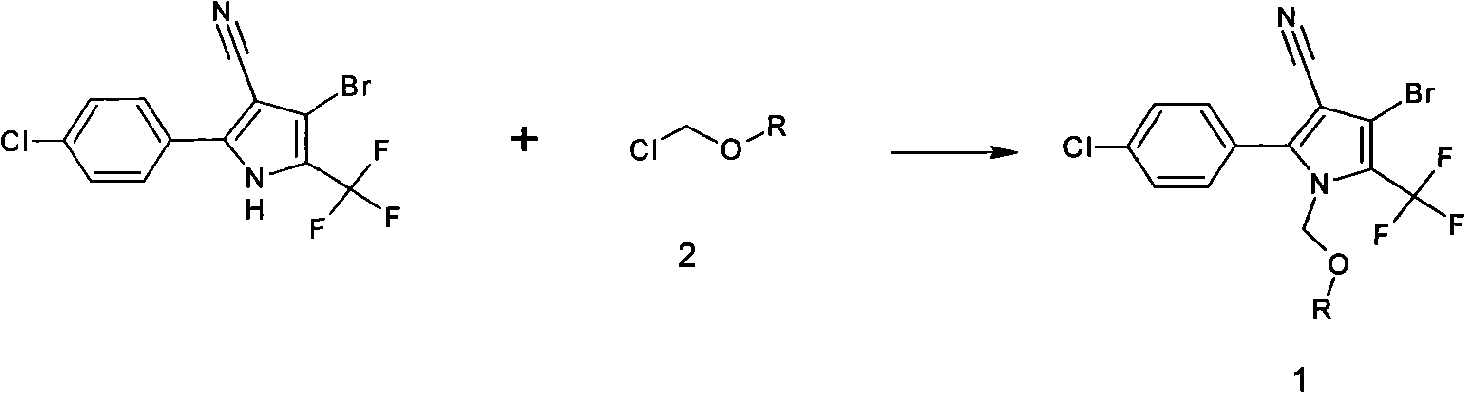
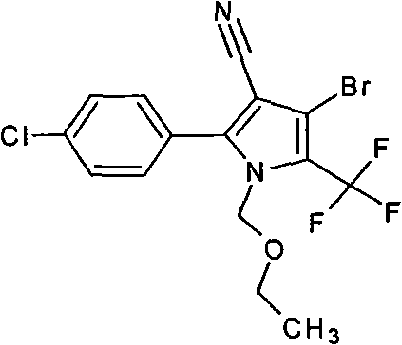
[0024] Add 35 grams of 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile, 8 grams of sodium hydroxide, and 200 ml of methyl isobutyl methyl ketone into the there-necked flask, stir for 20 minutes, Add 20 grams of chloromethyl ethyl ether dropwise, raise the temperature to 60°C, and react at this temperature for 2 hours, take a sample to monitor, the reaction is complete, cool down to room temperature, add 200ml of water, adjust the pH to <7 with 10% dilute hydrochloric acid, Layering, precipitation, and drying yielded 45.0 g of chlorfenapyr technical. Content 90%, yield 99%. 150ml of 80% tert-butanol was added to the precipitated material for recrystallization to obtain 38 grams of 98% chlorfenapyr as the original drug.
Embodiment 2
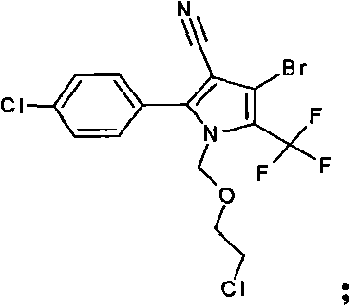
[0026] Add 35 grams of 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile, 12 grams of potassium hydroxide, and 200 ml of methyl isobutyl methyl ketone into the there-necked flask, and stir for 20 minutes. Add 20 grams of chloromethyl chloroethyl ether dropwise, raise the temperature to 55°C, and react at this temperature for 1 hour, take a sample to monitor, the reaction is complete, cool down to room temperature, add 200ml of water, and adjust the pH to 5 with 10% dilute hydrochloric acid , layered, precipitated, added 150ml of 80% ethanol for recrystallization, and obtained 42.0 g of the original drug of HNPC-A3061 with a content of 98%.
Embodiment 3
[0028] Add 35 grams of 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile, 9 grams of sodium hydroxide, and 200 ml of cyclohexanone into the three-necked flask, stir for 20 minutes, and add dropwise 20 gram of chloromethyl chloroethyl ether, warming up to 40°C, and reacting at this temperature for 3 hours, taking samples for monitoring, the reaction was complete, cooling down to room temperature, adding 200ml of water, adjusting the pH to 5 with 10% dilute hydrochloric acid, and layering , precipitation, adding 150ml of 80% methanol for recrystallization to obtain the original drug of HNPC-A3061.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com