Method for synthesizing key intermediate for preparing camptothecine compounds
A synthesis method and compound technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of no substantial breakthroughs in camptothecin process research, complex reaction operations, long synthesis routes, etc., and achieve the benefits of mass production , high stereoselectivity, and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
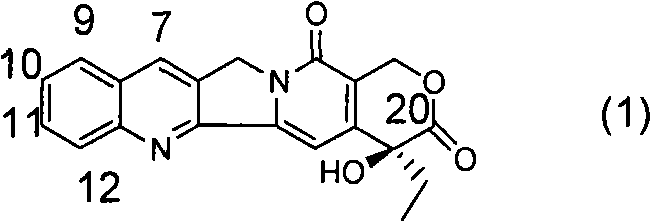
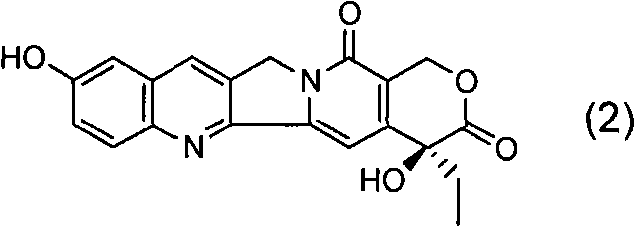
[0071] a. 1-{(4R)-2,2-Dimethyl-[1,3]dioxolan-4-yl}-1-[(1S)-ethyl]-4-methoxy-1H -Furan[3,4-c]pyridin-3-one
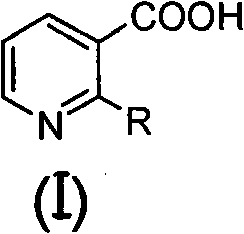
[0072] Under nitrogen protection, add 200ml of anhydrous THF, 14.4ml of 2,2,6,6-tetramethylpiperidine and 58.5ml of n-butyllithium (2.5M in n-hexane) solution into a 500ml three-necked flask, and cool to - After a period of reaction at 70°C, 100ml of anhydrous THF solution containing 5g of 2-methoxynicotinic acid was added to it, and the reaction was continued for a period of time, and 15.0g of (2R)-1,2-O- Mesopropylidenedioxy-3-pentanone, then moved to room temperature for reaction, after the reaction was completed, 100ml of 2N dilute hydrochloric acid was added to quench the reaction, after vigorous stirring, the liquid was left to separate, and the aqueous phase was extracted with 60ml×3 ethyl acetate , combined the organic phases, washed the organic phases with water until neutral, and then washed once with saturated brine, dried over anhydrous sodium sulfate, filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com