Synthesis method of ractopamine artificial antigen
A ractopamine and artificial antigen technology, applied in the field of ractopamine artificial antigen synthesis, can solve the problems of damaging the international reputation of export products, affecting the healthy development of animal husbandry, and repeatedly encountering green barriers to the export of livestock and poultry products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
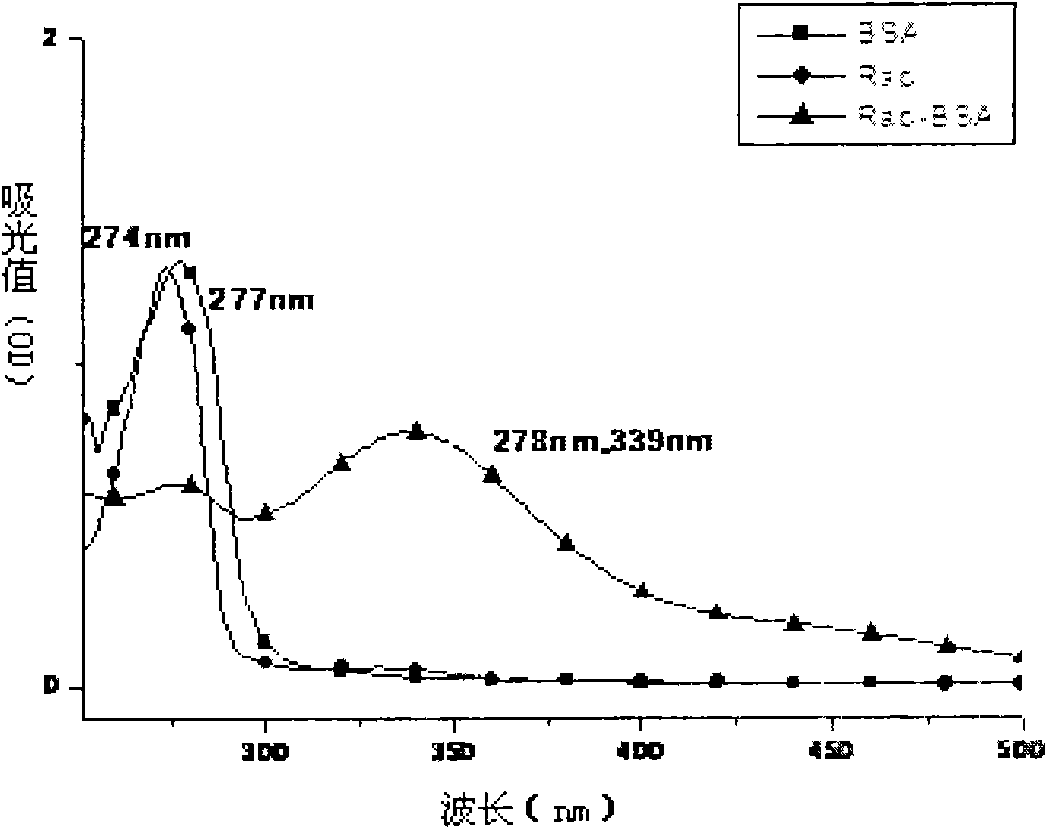
Image
Examples
Embodiment Construction
[0008] Synthetic method of ractopamine artificial antigen
[0009] (1) Preparation of activated p-aminobenzoic acid: take sodium nitrite and p-aminobenzoic acid in a ratio of 1:1 by molar ratio, dissolve sodium nitrite and p-aminobenzoic acid in distilled water and 0.2mol / L respectively In hydrochloric acid, stir in an ice bath, add the aqueous solution of sodium nitrite dropwise to the hydrochloric acid solution of p-aminobenzoic acid, and react at room temperature in the dark for 1 hour to obtain a colorless activated p-aminobenzoic acid solution.
[0010] The molar ratio range of sodium nitrite and p-aminobenzoic acid described in the present invention is 1~2: 1; The concentration range of described hydrochloric acid is 0.1~0.2mol / L, and the reaction time range of room temperature avoiding light is 0.5~1.5 hours .
[0011] (2) Preparation of active intermediates of ractopamine: take ractopamine at a ratio of 0.5:1 to the molar ratio of activated p-aminobenzoic acid, dissol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com