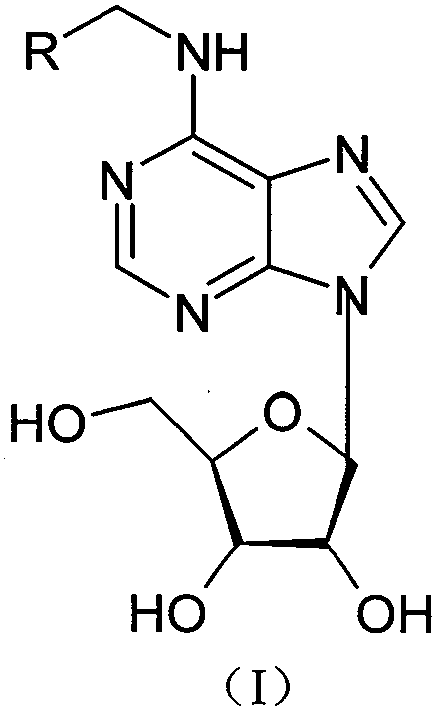
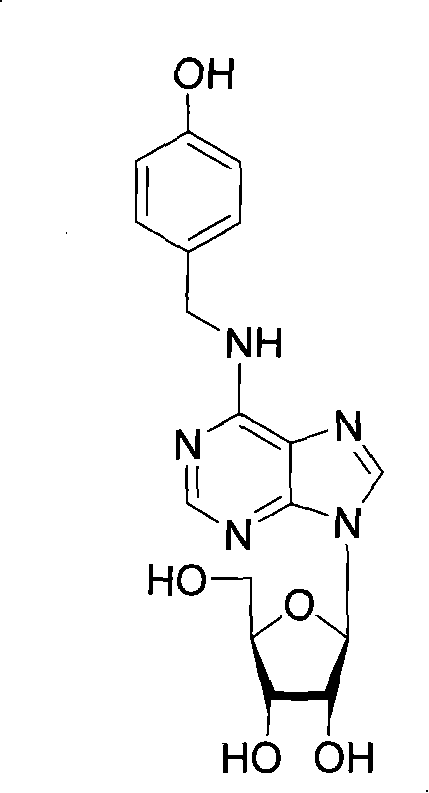
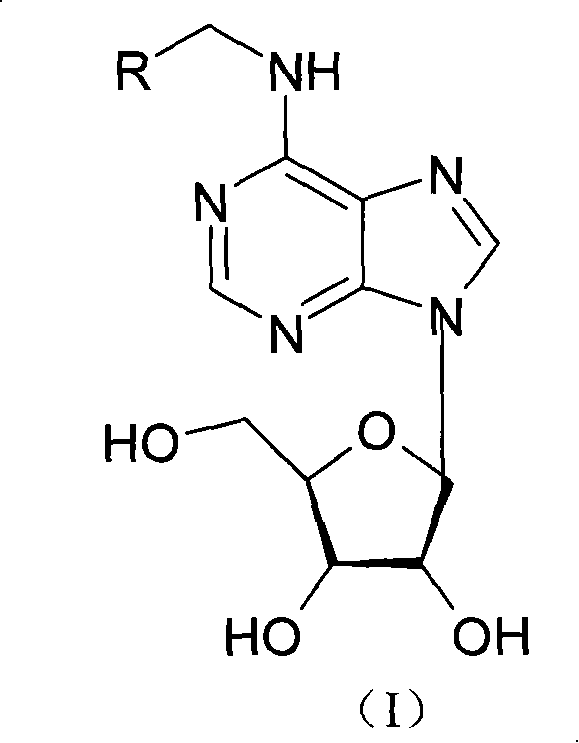
N<6>-substituted adenosine derivative, preparation method thereof, drug composition and application
A technology of adenosine derivatives and adenosine, which is applied in the field of medicine and can solve problems that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: N 6 Preparation of -(3,4-dihydroxybenzyl)-adenosine
[0066] In the first step, 2.76g of 3,4-dihydroxybenzaldehyde, 2.52g of hydroxylamine hydrochloride, and 3.26g of anhydrous sodium acetate were weighed and dissolved in 80ml of ethanol. The reaction was stirred for 6 hours at room temperature. The solvent was evaporated to dryness, dissolved in 40ml of water, extracted with ethyl acetate (40×3ml), and the ethyl acetate was evaporated to obtain 2.72g of light yellow solid (3,4-dihydroxybenzaldehyde oxime).
[0067] Second step, 2.72g 3,4-dihydroxybenzaldehyde oxime is dissolved in 70ml ethanol, adds 700mg 10%Pd / C, 8ml concentrated hydrochloric acid, hydrogenates under normal pressure, obtains white crystal (3,4-dihydroxybenzylamine Hydrochloride) 2.98g.
[0068] The third step, 2.98g 3,4-dihydroxybenzylamine hydrochloride was dissolved in 70ml n-propanol, add 1g 6-chloropurine nucleoside and 14ml N,N-diisopropylethylamine, heated to 70°C, react for 8 hou...
Embodiment 2
[0070] Example 2: N 6 Preparation of -(3-methoxy-4-hydroxybenzyl)-adenosine
[0071] In the first step, 3.04g of 3-methoxy-4-hydroxybenzaldehyde, 2.52g of hydroxylamine hydrochloride, and 3.26g of anhydrous sodium acetate were weighed and dissolved in 80ml of ethanol. The reaction was stirred for 6 hours at room temperature. The solvent was evaporated to dryness, dissolved in 40ml of water, extracted with ethyl acetate (40×3ml), and the ethyl acetate was evaporated to obtain 2.99g of light yellow solid (3-methoxy-4-hydroxybenzaldehyde oxime).
[0072] In the second step, 2.99g 3-methoxyl-4-hydroxybenzaldehyde oxime was dissolved in 70ml ethanol, added 700mg 10% Pd / C, 8ml concentrated hydrochloric acid, and hydrogenated at normal pressure to obtain white crystals (3-methoxyl- 4-hydroxybenzylamine hydrochloride) 3.31g.
[0073] In the third step, dissolve 3.31g of 3-methoxy-4-hydroxybenzylamine hydrochloride in 70ml of n-propanol, add 1g of 6-chloropurine nucleoside and 14ml ...
Embodiment 3
[0075] Example 3: N 6 Preparation of -(4-hydroxybenzyl)-adenosine
[0076]In the first step, 2.55g of 4-hydroxybenzaldehyde, 2.60g of hydroxylamine hydrochloride, and 3.40g of anhydrous sodium acetate were weighed and dissolved in 80ml of ethanol. The reaction was stirred for 6 hours at room temperature. The solvent was evaporated to dryness, dissolved in 40ml of water, extracted with ethyl acetate (40×3ml), and the ethyl acetate was evaporated to obtain 2.66g of light yellow solid (4-hydroxybenzaldehyde oxime).
[0077] In the second step, 2.66g of 4-hydroxybenzaldehyde oxime was dissolved in 70ml of ethanol, 300mg of 10% Pd / C was added, 8ml of concentrated hydrochloric acid was hydrogenated under normal pressure to obtain 3.02g of white crystals (4-hydroxybenzylamine hydrochloride) .
[0078] In the third step, dissolve 3.02g of 4-hydroxybenzylamine hydrochloride in 70ml of n-propanol, add 1g of 6-chloropurine nucleoside and 14ml of N,N-diisopropylethylamine, heat to 70°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com