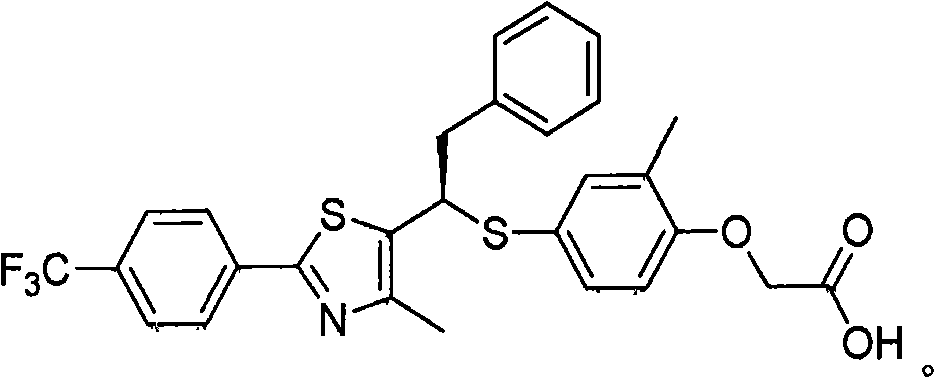
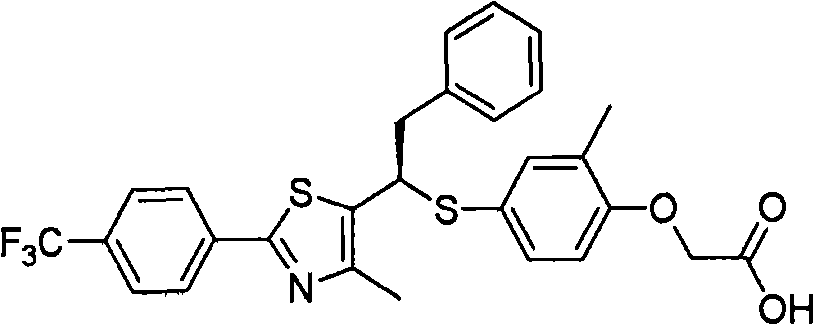
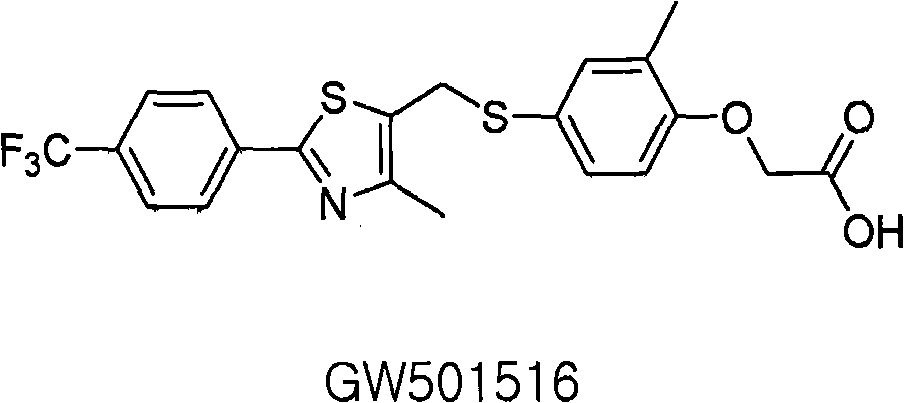
Thiazole compound (as ppard) ligand and pharmaceutical, cosmetic and health food comprised thereof
A compound, thiazole technology, applied in the fields of thiazole compounds as PPARδ ligands and pharmaceuticals, cosmetics and health foods containing them, can solve the problems that do not mention the pharmacological effects of selective activators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1: the preparation of the compound of formula 8 (step A)
[0093] 500 mg (2.14 mmol) of 4-iodo-2-methylphenol were dissolved in anhydrous tetrahydrofuran under nitrogen while maintaining the temperature at 0°C. 1.1 ml of isopropylmagnesium chloride (2M-diethyl ether solution, 2.16 mmol) was slowly added thereto, followed by reaction for 10 minutes. The reaction solution was cooled to -78°C, and 2.77 ml of tert-butyllithium (1.7M-heptane solution, 4.70 mmol) was slowly added thereto. After reacting for 20 minutes, 69 mg of S (2.14 mmol) was added thereto, and then the reaction was continued until the temperature of the reactant reached 15°C. After 40 minutes, 624 mg (2.14 mmol) of 5-chloromethyl-4-methyl-2-[(4-trifluoromethyl)phenyl]-thiazole represented by formula 7 was dissolved in 2 ml of anhydrous THF , at the same temperature (15 ° C) slowly added to the above reactants. After continuing the reaction for one hour, the reaction was terminated by adding ...
Embodiment 2
[0096] Embodiment 2: the preparation of the compound of formula 9 (R 1 =t-Bu(CH 3 ) 2 Si-, step B)
[0097] 500 mg (1.26 mmol) of the compound of formula 8 and 171 mg (2.52 mmol) of imidazole were completely dissolved in 5 ml of dimethylformamide. 209 mg (1.38 mmol) of tert-butyldimethylchlorosilane was slowly added thereto, followed by stirring at room temperature for 4 hours. After the reaction was complete, the organic solvent was extracted with ammonium chloride solution and ethyl acetate. The moisture in the organic phase was dried over magnesium sulfate. After filtration, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (hexane / ethyl acetate=10 / 1, v / v) to obtain 610 mg (yield: 95%) of the target compound.
[0098] 1 H NMR (300MHz, CDCl 3 ): 7.97(d, 2H, J=8.1Hz), 7.65(d, 2H, J=8.2Hz), 7.19(d, 1H, J=1.9Hz), 7.07(m, 1H), 6.69(d, 1H, J=8.3Hz), 4.11(s, 2H), 2.21(s, 3H), 2.11(s, 3H), 1.01(s, 9H), 0.2...
Embodiment 3
[0100] Embodiment 3: the preparation of the compound of formula 10 (R 1 =t-Bu(CH 3 ) 2 Si-, step C)
[0101] The compound (R 1 =t-Bu(CH 3 ) 2 -) was dissolved in 5 ml of anhydrous tetrahydrofuran in the presence of nitrogen and the temperature was lowered to -78°C. 619 µl (2.0 M ether solution, 1.24 mmol) of lithium diisopropylamide was slowly added thereto, followed by reaction for 10 minutes. Then, 77 µl (0.65 mmol) of benzyl bromide was added to the reaction solution, followed by stirring at the same temperature (-78°C) for 30 minutes. The reaction was terminated by adding 5 ml of ammonium chloride solution. The moisture in the organic phase was dried over magnesium sulfate. After filtration, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (hexane / ethyl acetate=10 / 1, v / v) to obtain 265 mg (yield: 75%) of the target compound.
[0102] 1H NMR (300MHz, CDCl 3 ): δ7.97(d, 2H, J=8.1Hz), 7.65(d, 2H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com