Synergic acaricide
An acaricide and solvent technology, applied in the field of pesticides, can solve the problems of poor quick-acting and environmental pollution, and achieve the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
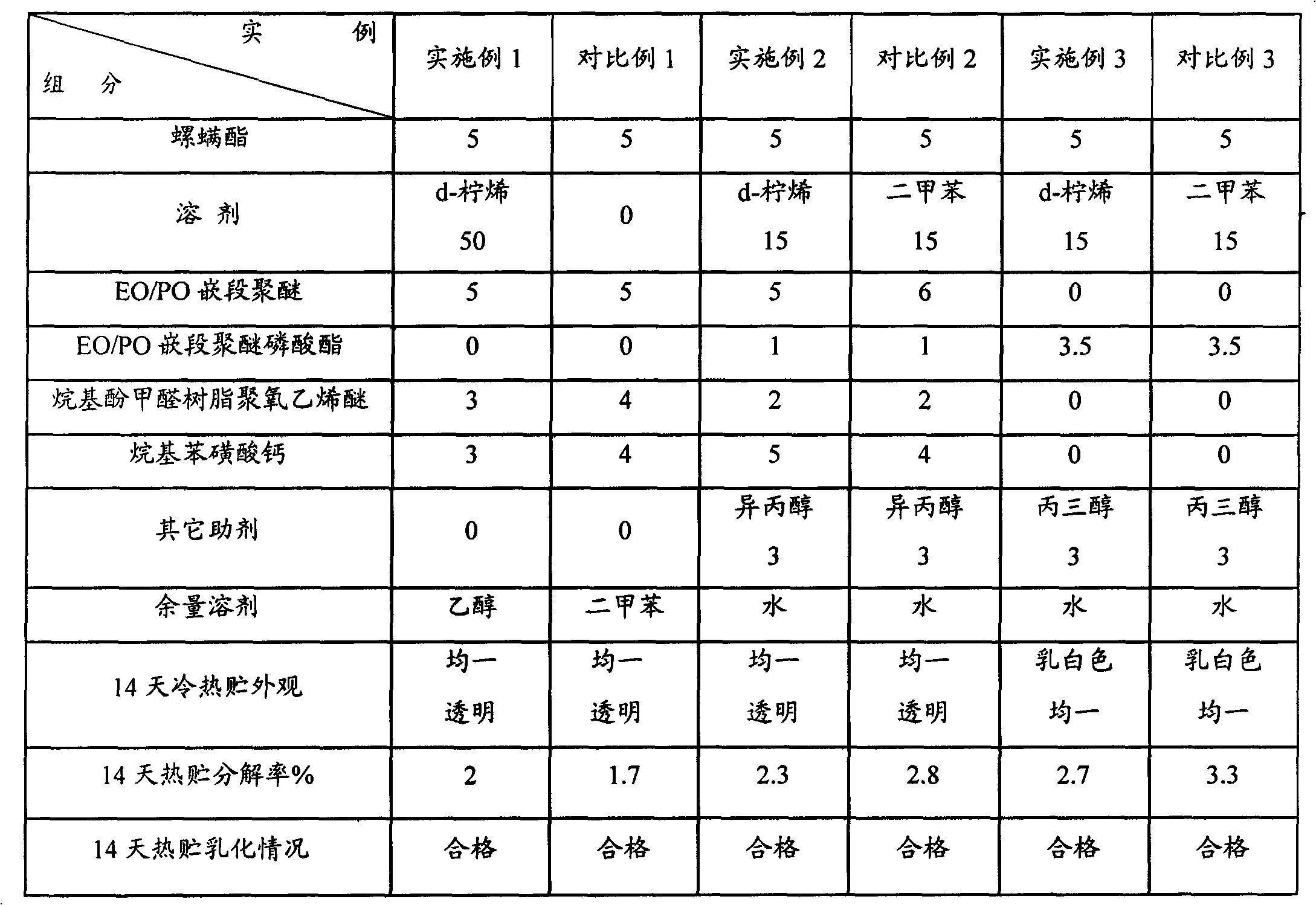
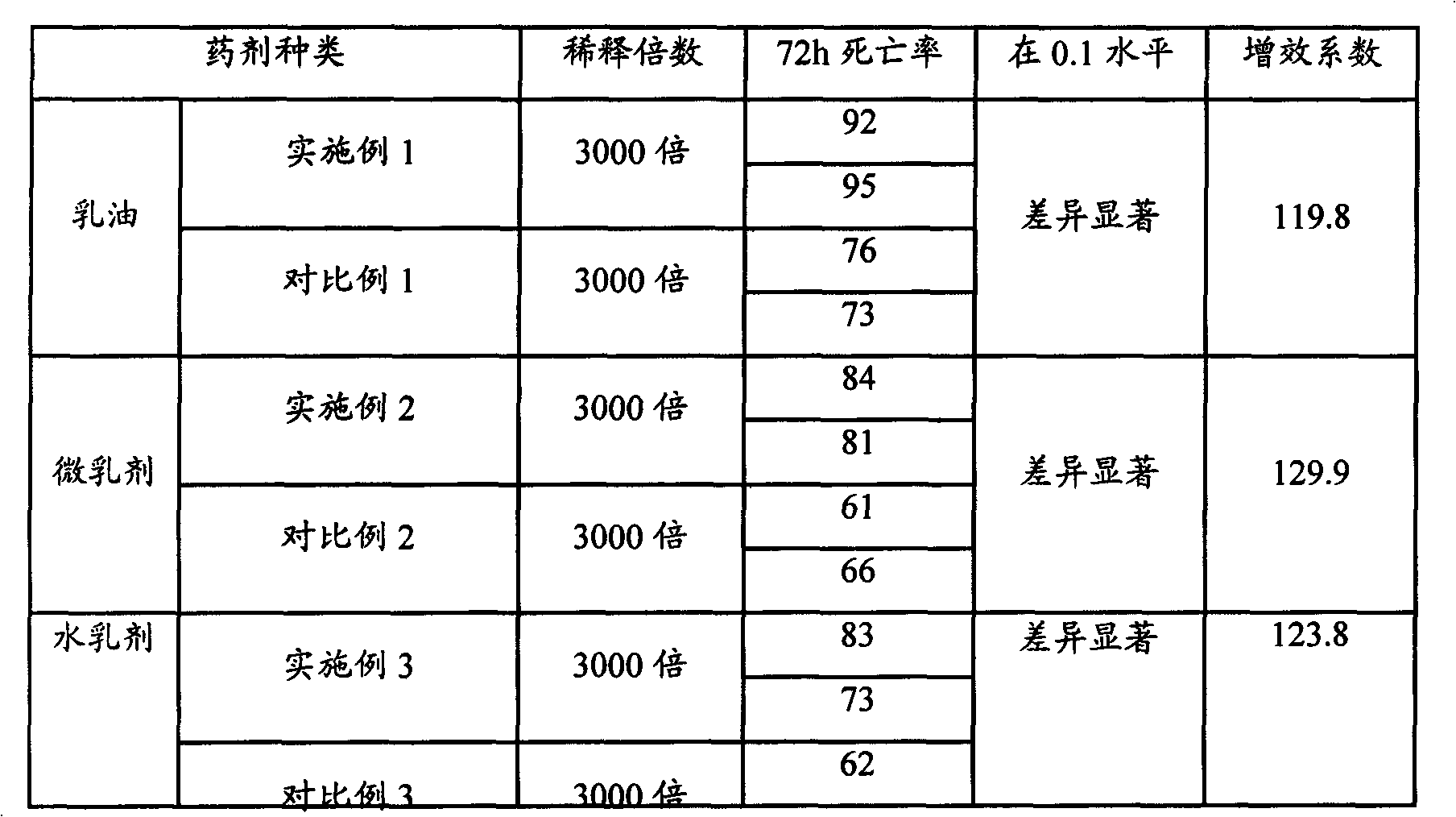
[0030] According to the formula of Example 1 in Table 1, first add d-limonene to the preparation kettle, then add spirodiclofen, stir for about 20 minutes to dissolve completely, add emulsifier and make up the solvent, continue to stir evenly for about 15 minutes to prepare 5% For the spirodiclofen emulsifiable concentrate preparation, the content of each component in the formula is calculated by mass percentage. The prepared preparations were sealed in 10ml ampoules, and 2 sealed bottles of each agent were stored in a refrigerator at 0°C and an oven at 54°C for 14 days, and the content was analyzed at the end of the day to obtain the decomposition rate. The results are shown in Table 1.
Embodiment 2
[0034] According to the formula of Example 2 in Table 1, first add d-limonene in the preparation kettle, then add spirodiclofen, stir and dissolve completely for about 20 minutes, then add emulsifier and continue stirring for about 10 minutes, and continue stirring for about 10 minutes after making up with water. A homogeneous and transparent liquid was obtained in 15 minutes, which was prepared into 5% spirodiclofen microemulsion. The prepared preparations were sealed in 10ml ampoules, and 2 sealed bottles of each agent were stored in a refrigerator at 0°C and an oven at 54°C for 14 days, and the content was analyzed at the end of the day to obtain the decomposition rate. The results are shown in Table 1.
Embodiment 3
[0038] According to the formula of Example 3 in Table 1, first add d-limonene in the preparation kettle, then add spirodiclofen, stir and dissolve completely for about 20 minutes, then add emulsifier and continue to stir evenly for about 10 minutes to complete the preparation of the oil phase. Add water into the mixing tank, and slowly add the oil phase under stirring, so that the oil phase is dispersed in the water phase to form a milky white water emulsion, add antifreeze and stir evenly to prepare a 5% spirodiclofen water emulsion. The prepared preparations were sealed in 10ml ampoules, and 2 sealed bottles of each agent were stored in a refrigerator at 0°C and an oven at 54°C for 14 days, and the content was analyzed at the end of the day to obtain the decomposition rate. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com