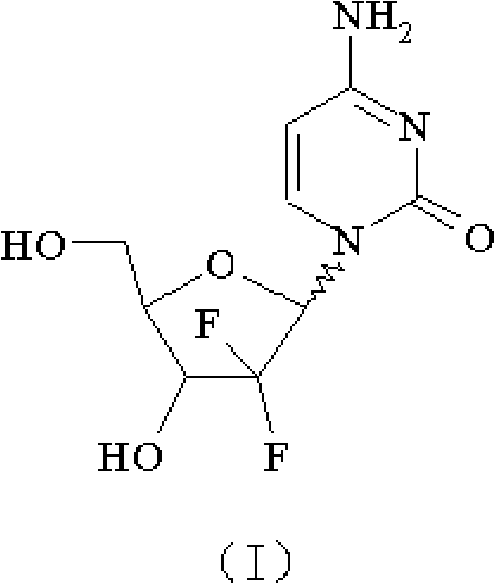
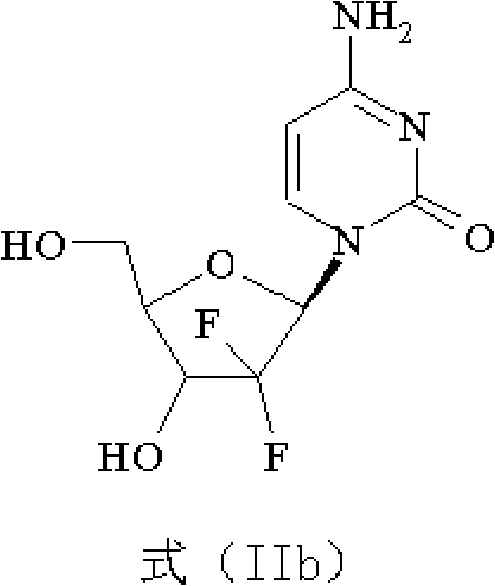
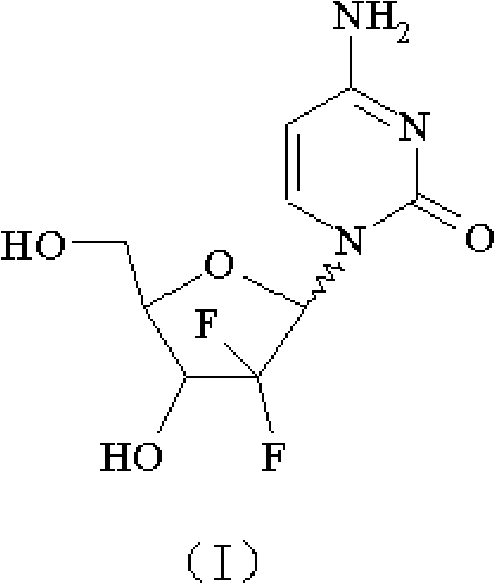
Gemcitabine hydrochloride composition and preparation method thereof
A technique for gemcitabine hydrochloride and its composition, which is applied in the field of gemcitabine hydrochloride composition and preparation, and can solve the problems of poor stability of gemcitabine hydrochloride, troublesome storage and transportation of liquid reagents, and easy decomposition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Hydrolysis: Add 100 g (212.3 mmol, α / β=1.1) of 2'-deoxy-2', 2'-difluorocytidine-3', 5'-dibenzoate into methanol, cool down to 7-8 ℃, feed 30g of ammonia gas to react, after the completion of the reaction, the temperature is naturally raised to room temperature, stirred for 8h, and then concentrated to near dryness, the obtained product is dissolved in 2150ml of water, extracted by adding 2150ml of ethyl acetate after dissolution, the water layer is separated and then used with 2150ml of ethyl acetate Extract, combine ethyl acetate, extract with 2150ml of water, combine all the water layers, decolorize with activated carbon, filter the obtained aqueous solution and concentrate under reduced pressure (pressure 1330Pa, temperature 60°C) to nearly dry, add 2150ml of isopropanol to the concentrate, heat to 70 ℃, heat preservation for 0.5h, and then heat preservation at 30°C for 7.5h, then cool down to about 0°C, freeze for 2h, and filter to obtain 51.2g of white solid of form...
Embodiment 2
[0097] Hydrolysis: Add 200 g (424.6 mmol, α / β=1.05) of 2'-deoxy-2', 2'-difluorocytidine-3', 5'-dibenzoate into methanol, cool down to 8-10 ℃, feed 85.7g of ammonia gas to react, after the completion of the reaction, the temperature is naturally raised to room temperature, stirred for 8h, and then concentrated to near dryness, the obtained product is dissolved in 4300ml of water, and extracted by adding 4300ml of ethyl acetate after dissolution, the water layer is separated and then used with 4300ml of ethyl acetate Ester extraction, combined with ethyl acetate, extracted with 4300ml of water, combined all the water layers, decolorized with activated carbon, concentrated the aqueous solution obtained by filtration under reduced pressure (pressure 4030Pa, temperature 55°C) to near dryness, added 4300ml of isopropanol to the concentrate, and heated to 50°C, keep warm for 0.4h, then keep warm at 28°C for 7.5h, then cool down to about -5°C, freeze for 2h, filter to obtain 101.6g of ...
Embodiment 3
[0101] Hydrolysis: Add 50 g (106.15 mmol, α / β=1.03) of 2'-deoxy-2', 2'-difluorocytidine-3', 5'-dibenzoate into methanol, cool down to 5-7 ℃, feed 11.5g of ammonia gas to react, after the completion of the reaction, the temperature is naturally raised to room temperature, stirred for 10h, and then concentrated to nearly dryness, the obtained product is dissolved in 1075ml of water, extracted by adding 1075ml of ethyl acetate after dissolution, the water layer is separated and then used in 1075ml of ethyl acetate Ester extraction, combined ethyl acetate, extracted with 1075ml of water, combined all the water layers, decolorized with activated carbon, concentrated the aqueous solution obtained by filtration under reduced pressure (pressure 1000Pa, temperature 40°C) to nearly dryness, added 900ml of isopropanol to the concentrate, and heated To 90°C, keep warm for 0.6h, then keep warm at 33°C for 7.0h, then cool down to about -5~-2°C, freeze for 2h, filter to obtain 25.4g of white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com