Method for renaturing and purifying recombinant extremely heat-resistant alpha-amylase
A technology of amylase and thermophilic archaea, applied in the biological field, can solve the problem of high operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
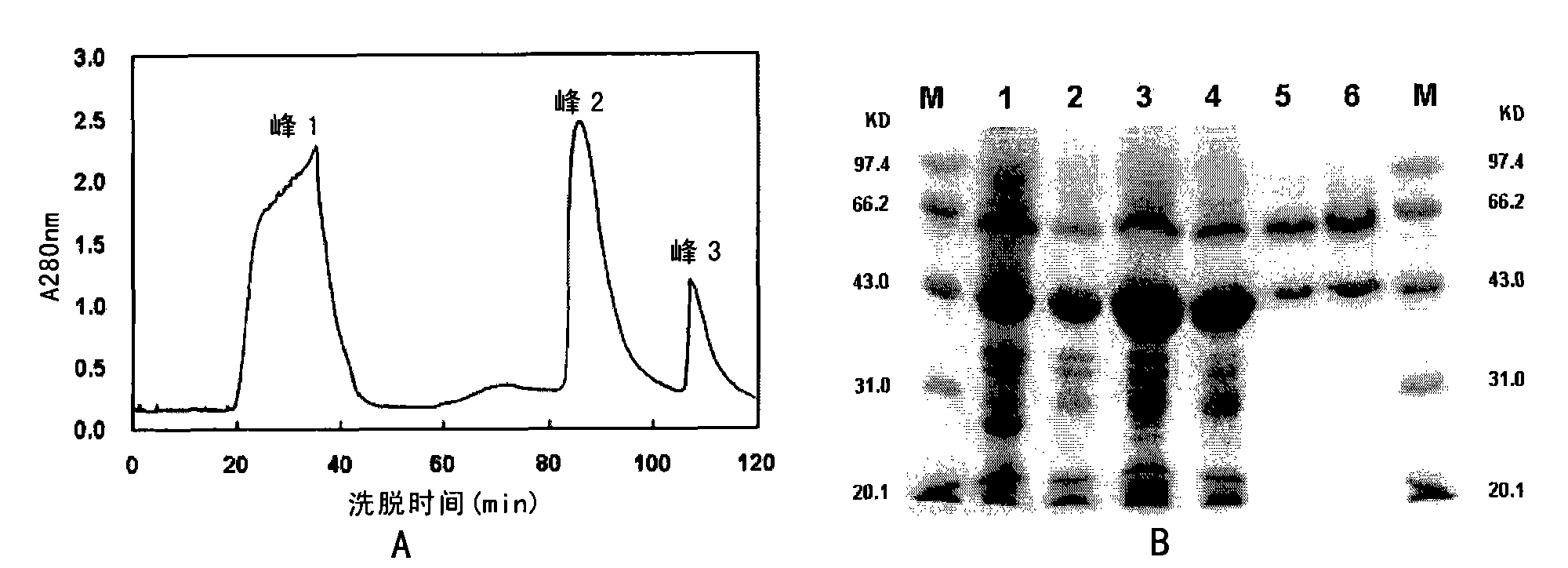
[0047] Therefore, the present invention provides a kind of preparation method of thermophilic α-amylase, it comprises the steps: (1) dissolve the inclusion body of thermophilic α-amylase with detergent solution, thereby obtain the inclusion body containing thermophilic α-amylase solution; and (2) isolating said thermophilic alpha-amylase from the solution obtained in (1).
[0048] The thermophilic α-amylase that can be used in the present invention can be from the thermophilic α-amylase of thermophilic archaea (also translated as Pyrococcus furiosus), the thermophilic α-amylase of Pyrococcus genus, Pyrococcus worriii ( Pyrococcus woesei) and thermophilic α-amylases from other thermophilic microorganisms. In the present invention, the thermophilic α-amylase may also be a variant of the thermophilic α-amylase. Representative thermophilic alpha-amylases include, but are not limited to, those encoded by the following genes: GenBank Accession Nos. U96622, AF001268, AF177906.1, AE0...
Embodiment 1
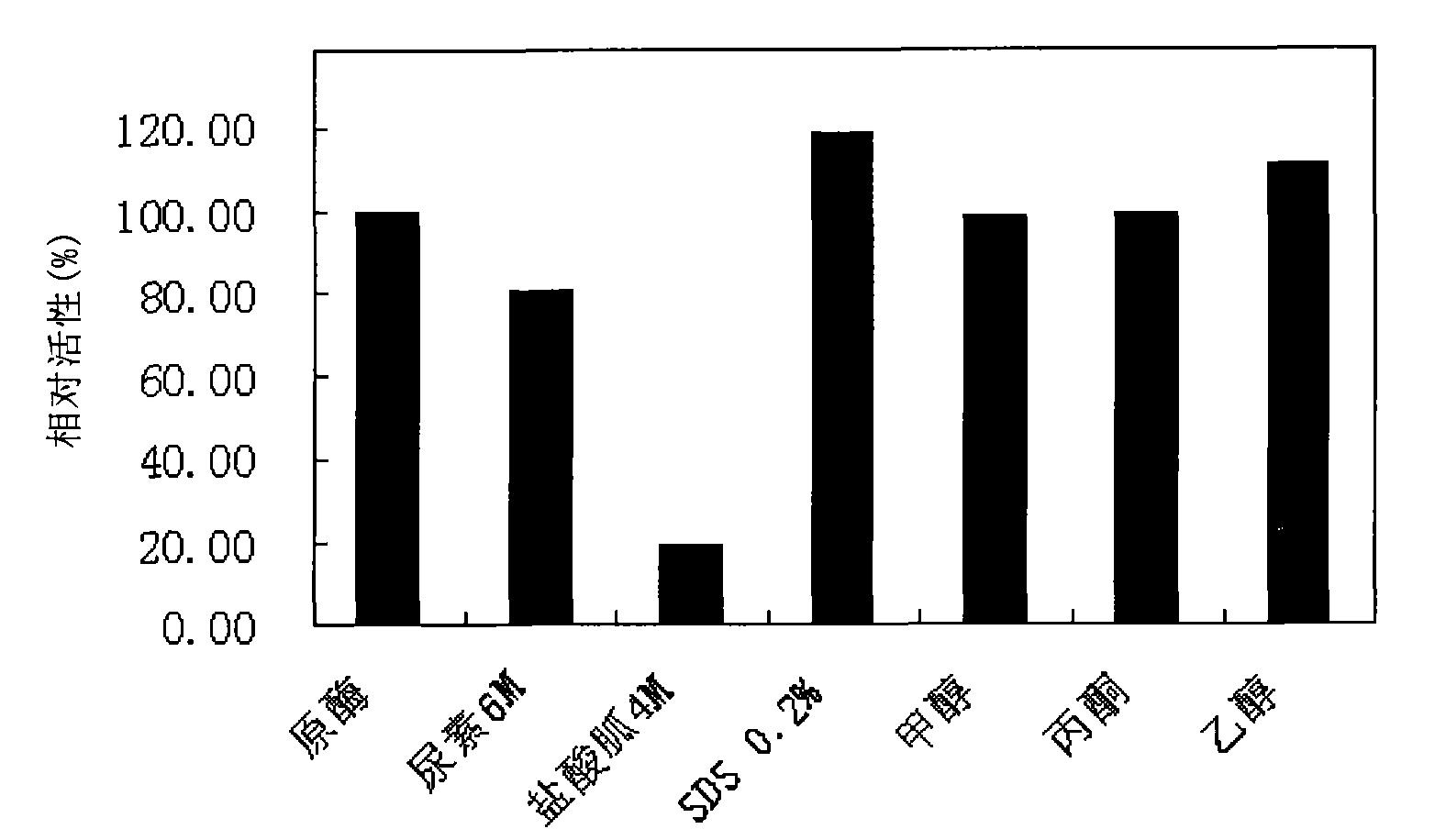
[0093] The influence of embodiment 1 denaturing agent and organic solvent on PFA amylase activity
[0094] As previously reported PFA has strong stress resistance. The present inventor has studied the influence of several denaturants and organic solvents on the activity of PFA enzyme. PFA amylase was placed in 6M urea, 4M guanidine hydrochloride, 0.2% SDS, and 1 / 8 (V / V) methanol, acetone, and ethanol to measure its enzyme activity. It was found that 4M guanidine hydrochloride would reduce the enzyme activity. Three organic solvents Little effect on the enzyme activity of PFA. However, SDS not only has no effect on the enzyme activity of PFA, but also enhances it to a certain extent. The results are shown in figure 1 .
Embodiment 2
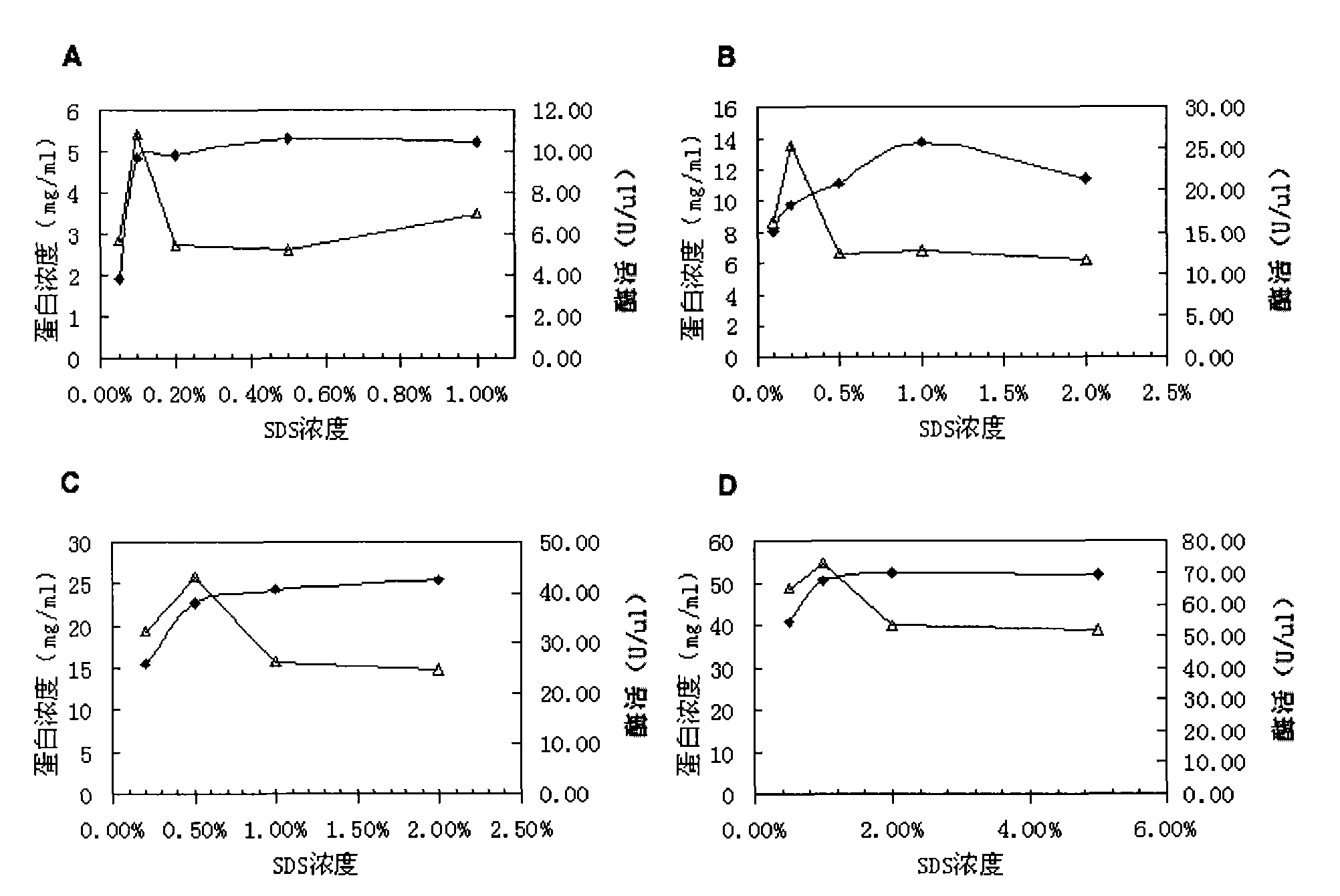
[0095] Embodiment 2 Solubility of inclusion bodies in different concentrations of detergents
[0096] SDS has been used for the solubilization of poorly soluble proteins. According to the aforementioned verification, the detergent will not inhibit the amylase activity of PFA, so the inventors tried to use the detergent to dissolve the PFA inclusion body in order to obtain a simpler and more efficient purification method.
[0097] By measuring the solubility of several concentrations of inclusion bodies in different concentrations of detergents, it is found that as the concentration of inclusion body protein increases, the concentration of detergent required to fully dissolve inclusion bodies increases, see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com