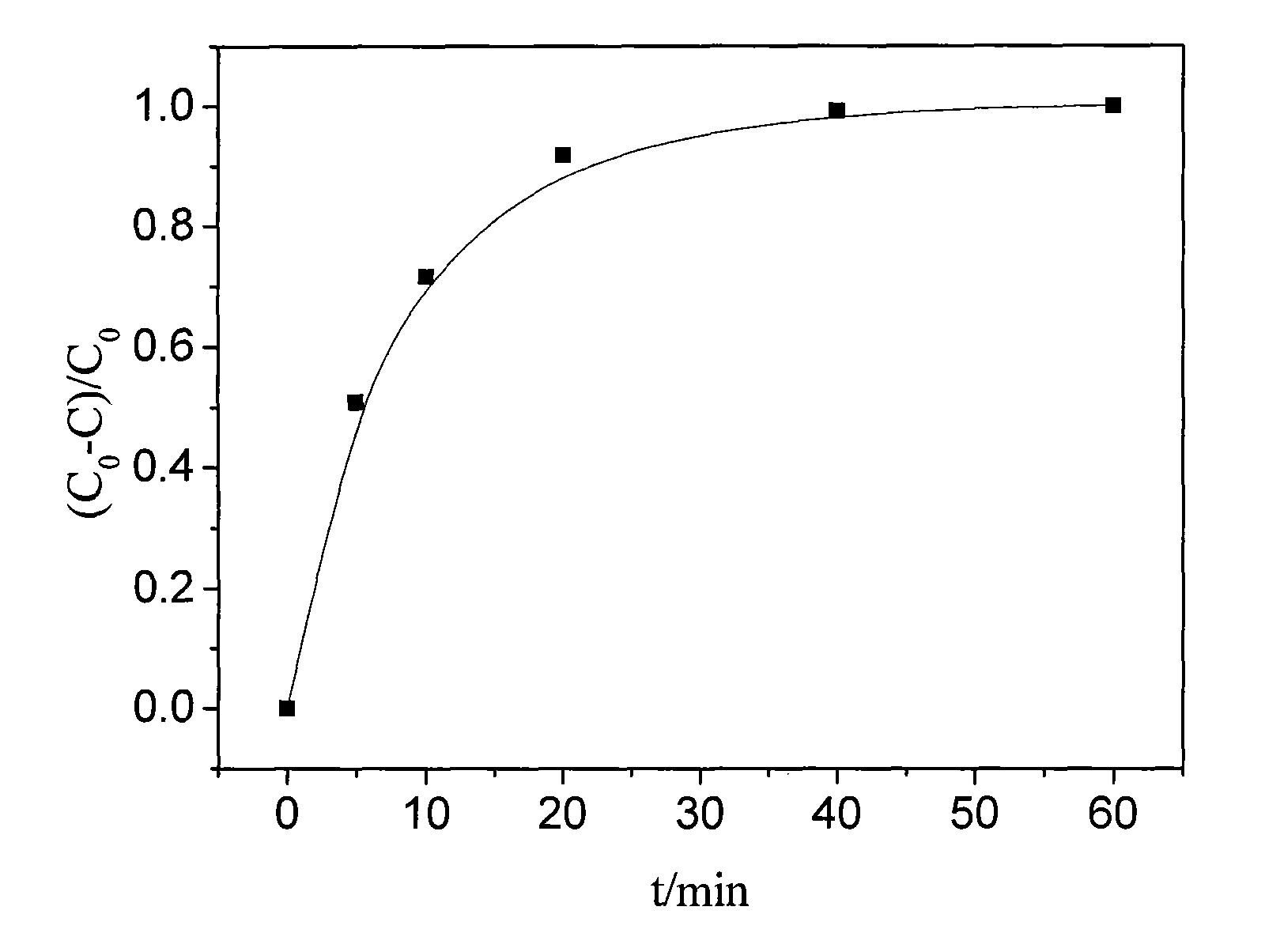
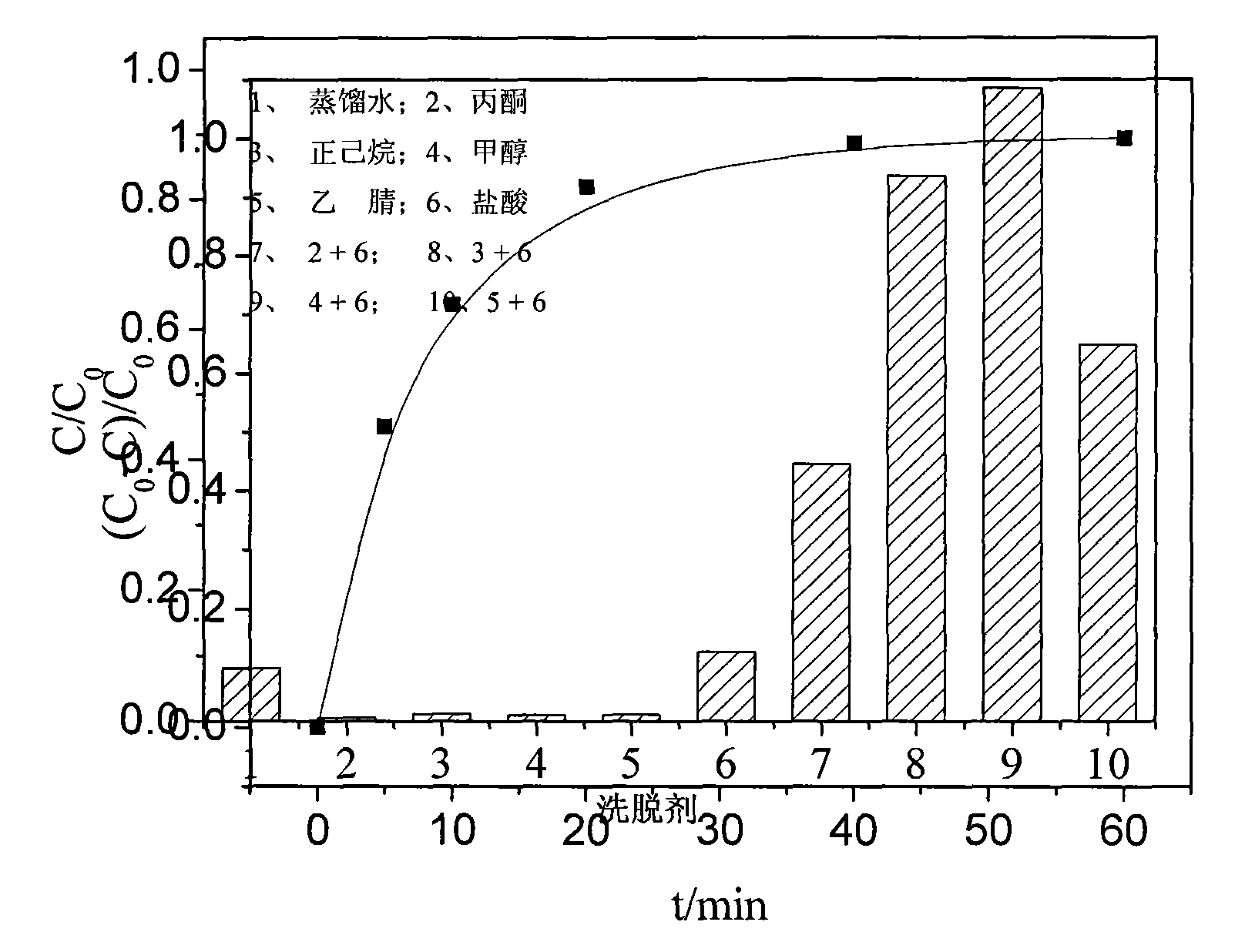
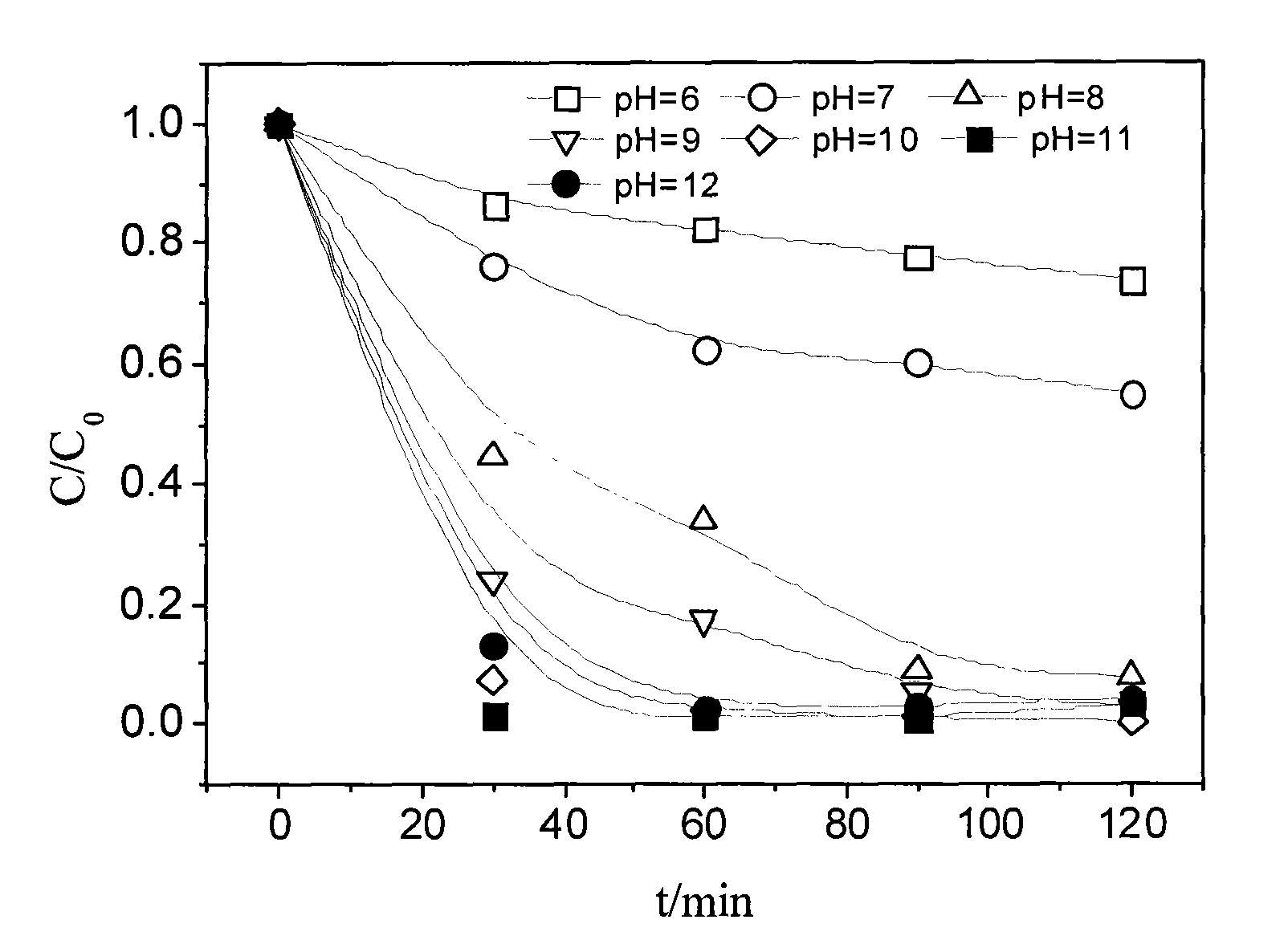
Method of catalyzing hydrogen peroxide with molybdate-carried ion exchange resin to degrade trichlorophenol
A technology of ion exchange resin and hydrogen peroxide, applied in chemical instruments and methods, oxidized water/sewage treatment, water pollutants, etc., can solve the problems of degradation efficiency reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] A. Experimental part:
[0021] 1) Instruments and reagents:
[0022] An Agilent 6890N gas chromatograph is equipped with an ECD detector to determine the concentration of trichlorophenol in the degradation system, and the chromatographic column is a DB-5 chromatographic column (60m×0.25mm, 25μm). The inlet is in splitless mode, the temperature is 250°C; the purge flow rate is 1mL min -1 , Purge time: 0.5min. The initial temperature is 150°C, keep for 3min; at 30°C·min -1 The speed was raised to 300°C and kept for 10 minutes. The detector temperature was 330°C.
[0023] The solid-phase microextraction handle and polydimethylsiloxane (PDMS, 100 μm) extraction head are products of Supelco Inc (Bellefonte, PA, USA). Trichlorophene and trimethyl hydroxysulfide were purchased from Bailingwei Company without any purification steps before use. Sodium molybdate and hydrogen peroxide were purchased from Henan Jiaozuo Chemical Reagent Company, and 717 strong basic styrene an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com