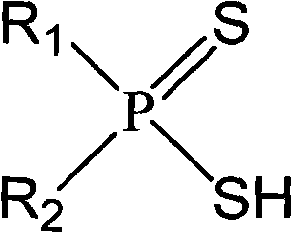
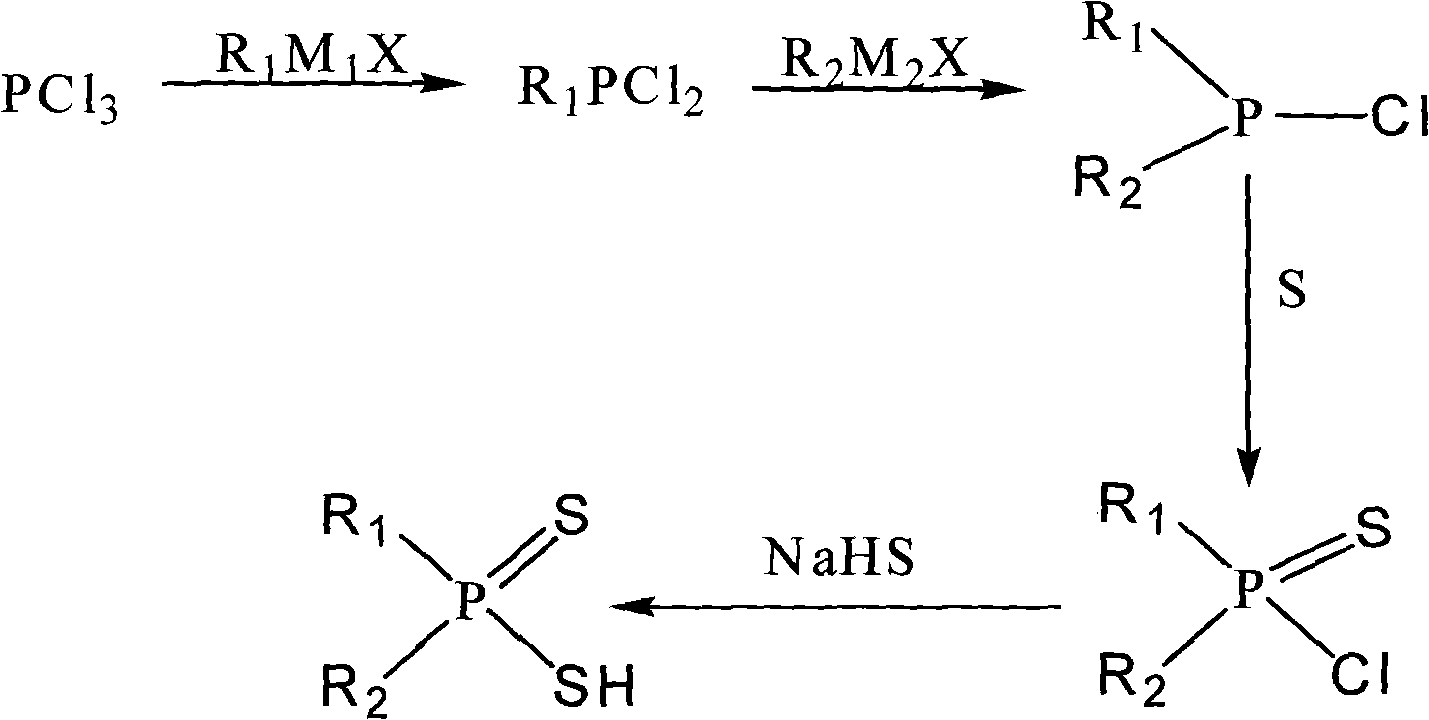
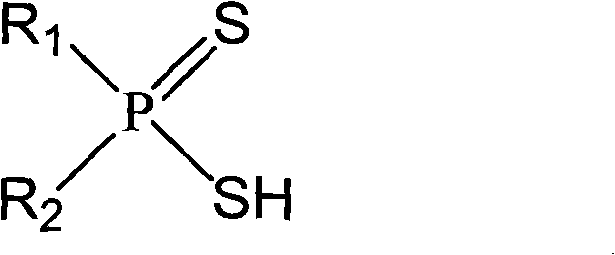
Asymmetric dithiophosphinic acid synthesis method
A technology of dithiophosphinic acid and synthesis method, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry and other directions, and can solve problems such as lack of synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 o-trifluoromethylphenyl (octyl) dithiophosphinic acid
[0045] The first step, preparation of organometallic reagent ①
[0046] In this example, organometallic reagent ① uses organozinc reagent;
[0047] (1), preparation of Grignard reagent octylmagnesium bromide
[0048] Under the protection of argon, add 0.525mol magnesium strips Mg12.778g and anhydrous ether 25mL into the three-neck flask according to the weight volume ratio of 1:1.95, then add 0.05g of iodine particles; add 2~3mL of n-octyl bromide Alkane C 8 h 17 Br (87.0mL, 0.5mol) ether (275mL) solution to the three-necked reaction flask to initiate the reaction, n-octane bromide C 8 h 17 The volume ratio of Br to anhydrous ether is 1:3.16; while stirring, add bromo-n-octane C 8 h 17 Br ether solution, after the dropwise addition is completed, continue to heat to 30-40°C and reflux until the magnesium bars disappear;
[0049] (2), the obtained brown-black clear liquid, that i...
Embodiment 2
[0065] The synthesis of embodiment 2 butyl octyl dithiophosphinic acid
[0066] The first step, preparation of organometallic reagent ①
[0067] In this example, organometallic reagent ① uses organozinc reagent;
[0068] (1), preparation of Grignard reagent octylmagnesium bromide
[0069] Under the protection of argon, add 0.42 mol of magnesium strips Mg10.0g and anhydrous ether 10mL into the three-neck flask according to the weight volume ratio of 1:1, then add 0.05g of iodine particles; add 2~3mL of n-octyl bromide Alkane C 8 h 17 Br (69.4mL, 0.4mol) ether (210mL) solution to the three-necked flask to initiate the reaction, n-octane bromide C 8 h 17 The volume ratio of Br to anhydrous ether is 1:3.04; while stirring, add brominated n-octane C 8 h 17 Br ether solution, after the dropwise addition is completed, continue to heat to 30-40°C and reflux until the magnesium bars disappear;
[0070] (2), the obtained brown-black clear liquid, that is, the Grignard reagent, w...
Embodiment 3
[0086] The synthesis of embodiment 3 o-trifluoromethylphenyl (phenyl) dithiophosphinic acid
[0087] The first step, organometallic reagent ① preparation
[0088] In this example, organometallic reagent ① uses Grignard reagent;
[0089] Preparation of Grignard Reagent Phenylmagnesium Bromide
[0090] Under the protection of argon Ar, add 0.105mol magnesium strips Mg2.553g and anhydrous ether 5mL into the three-neck flask according to the weight volume ratio of 1:1.99, then add 0.05g of iodine particles; dropwise add 2~3mL of bromobenzene C 6 h 5 Br (10.5mL, 0.1mol) diethyl ether (35mL) solution to the three-necked flask to initiate the reaction, bromobenzene C 6 h 5 The volume ratio of Br to anhydrous ether is 1:3.33; add bromobenzene C dropwise while stirring 6 h 5 Br ether solution, after the dropwise addition is completed, continue to heat to 30-40°C and reflux until the magnesium bars disappear;
[0091] The second step, phenyl phosphorus dichloride C 6 h 5 PCl 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com