Preparation method of fipronil formed by oxidization of phenyl pyrazole derivatives
A technology of phenylpyrazole and its derivatives, which is applied in the field of preparation of fipronil, can solve the problems of high moisture content, poor reaction selectivity, and high ratio of by-product sulfuryl compounds in the recovery of trifluoroacetic acid, and achieve good industrial application prospects, reaction The effect of mild conditions and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
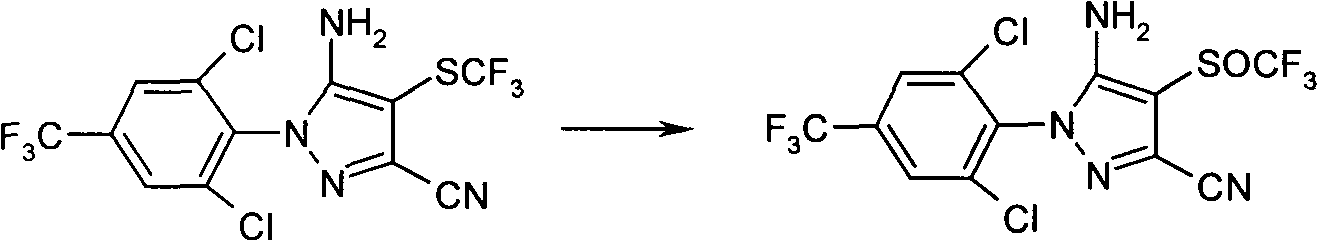
[0017] Add 40 grams of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoro Methylthiopyrazole, 150ml of formic acid with a volume concentration of 60-90%, stirred for 20 minutes, added 0.2 grams of vanadium pentoxide, slowly added 12 grams of hydrogen peroxide with a volume concentration of 20-60%, and controlled the temperature at 9-13 ℃, and react at this temperature for 5-6 hours. Sampling was done for HPLC detection. After the reaction was completed, 2.1 g of anhydrous sodium sulfite was added to continue the reaction for 1 hour. At a temperature of 30 to 50 ° C, the formic acid was evaporated under reduced pressure (vacuum degree of 0.04 to 0.095 MPa), 50 ml of methanol was added, and mole 10% aqueous sodium hydroxide solution to neutralize residual formic acid. Filtrate, take the precipitated solid, wash with water until neutral, and dry to obtain 38.2 g of crude fipronil.
[0018] Mix toluene and n-hexane at a volume ratio of 4:1 to form a mixed solve...
Embodiment 2
[0022] Under the temperature environment of -10 ~ 60 ℃, slowly add 24 grams of solid sodium perborate or solid sodium perborate and its hydrate, 0.2 grams of vanadium pentoxide into 60 grams of 80% formic acid, stir for 20 minutes, at 9 40 g of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyridine dissolved in 150 ml of formic acid was added dropwise at ~13°C azole, and react at this temperature for 2 to 3 hours. Sampling for HPLC detection. After the reaction is completed, add 2.1 g of anhydrous sodium sulfite to continue the reaction for 1 hour. At a temperature of 30 to 50 ° C, evaporate the formic acid under reduced pressure (vacuum degree of 0.04 to 0.095 MP), add 50 ml of methanol, and slowly add the molar concentration 10% aqueous sodium hydroxide solution to neutralize residual formic acid. Filtrate, take the precipitated solid, wash with water until neutral, and dry to obtain 37.5 g of crude fipronil with a content of 93%.
Embodiment 3
[0024] Add 40 grams of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoro Methylthiopyrazole, 0.2 g of vanadium pentoxide and 150 ml of 80% formic acid were stirred and dissolved. Add 24.4 grams of m-chloroperoxybenzoic acid three times in the range of 9 to 13 ° C, and react at this temperature for 8-12 hours, take a sample to monitor HPLC, after the reaction is completed, add 2.1 grams of anhydrous sodium sulfite to continue the reaction for one hour, At a temperature of 30-50°C, distill the formic acid under reduced pressure (vacuum degree 0.04-0.095MP), add 50ml of methanol, and slowly add 10% sodium hydroxide solution to neutralize the remaining formic acid. Filter, wash the precipitated solid with water until neutral, and dry to obtain 38.1 g of crude fipronil with a content of 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com