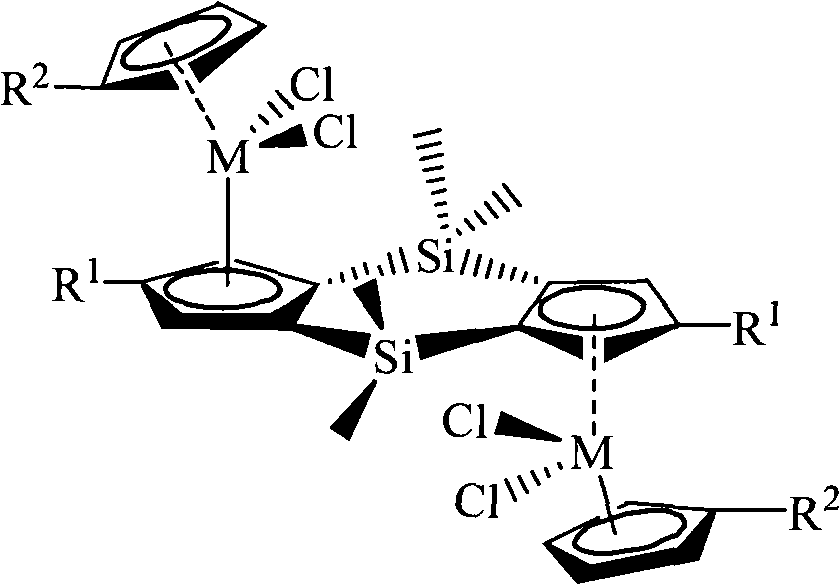
Bis-silicon-bridged metallocene compound, preparation method and application thereof
A technology of metallocene compound and silicon bridge, which is applied in the field of double silicon bridge metallocene compound, can solve the problem of no substituent group in the bridged ring, and achieve the effect of high olefin polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Ligand L1{μ,μ-[(Me 2 Si) 2 (η 5 -AllylC 5 h 2 ) 2 ]}Synthesis
[0058] Add 50mL (0.42mol) (CH 3 ) 2 SiCl 2 , and then add 100 mL of n-hexane, and stir evenly. Add 260 mL (0.84 mol) of 1.6 M CpNa dropwise under ice-water bath conditions, react for 6 hours, add 50 mL of distilled water to wash, use a pear-shaped separatory funnel to separate liquid to remove the organic phase, wash the water phase with 3 × 20 mL of ether, and combine the organic phases , and with anhydrous MgSO 4 Dry, filter and remove solvent in vacuo. Distill under reduced pressure, collect fractions at 40-42°C / 2mmHg, and obtain 18.05 g of a light yellow liquid product; add the above product to a 250 mL Schlenk bottle, and dissolve with 80 mL of n-hexane. Add about 53mL of 1.8M n-BuLi dropwise under ice-water bath, stir the reaction for 4 hours, then add 11.6mL (CH 3 ) 2 SiCl 2 and 20mLTHF, stirred overnight. Precipitate after completion of the reaction, filter the supernatant, concentrat...
Embodiment 2
[0060] Ligand L2{μ,μ-[(Me 2 Si) 2 (η 5 -BuC 5 h 2 ) 2 ]}Synthesis
[0061] Add 50mL (0.42mol) (CH 3 ) 2 SiCl 2 , and then add 100 mL of n-hexane, and stir evenly. Add 260 mL (0.84 mol) of 1.6 M CpNa dropwise under ice-water bath conditions, react for 6 hours, add 50 mL of distilled water to wash, use a pear-shaped separatory funnel to separate liquid to remove the organic phase, wash the water phase with 3 × 20 mL of ether, and combine the organic phases , and with anhydrous MgSO 4 Dry, filter and remove solvent in vacuo. Distill under reduced pressure, collect fractions at 40-42°C / 2mmHg, and obtain 18.05 g of a light yellow liquid product; add the above product to a 250 mL Schlenk bottle, and dissolve with 80 mL of n-hexane. Add about 53mL of 1.8M n-BuLi dropwise under ice-water bath, stir the reaction for 4 hours, then add 11.6mL (CH 3 ) 2 SiCl 2 and 20mLTHF, stirred overnight. Precipitate after completion of the reaction, filter the supernatant, concentrate u...
Embodiment 3
[0063] Ligand L3{μ,μ-[(Me 2 Si) 2 (η 5 -Me 3 SiC 5 h 2 ) 2 ]}Synthesis
[0064] Also press embodiment 1 method, use Me 3 SiCl replaces allyl bromide, and the ligand L3{μ,μ-[(Me 2 Si) 2 (η 5 -Me 3 SiC 5 h 2 ) 2 ]} white solid 52mmol, product yield 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com