Naphthol skeleton phenol-phosphine neutral nickel catalyst preparation method and application in preparing ethylene/vinyl polar monomer co-polymer
A nickel catalyst and catalyst technology, applied in the direction of nickel organic compounds, etc., can solve problems such as unsuitable for industrialization and expensive metal palladium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
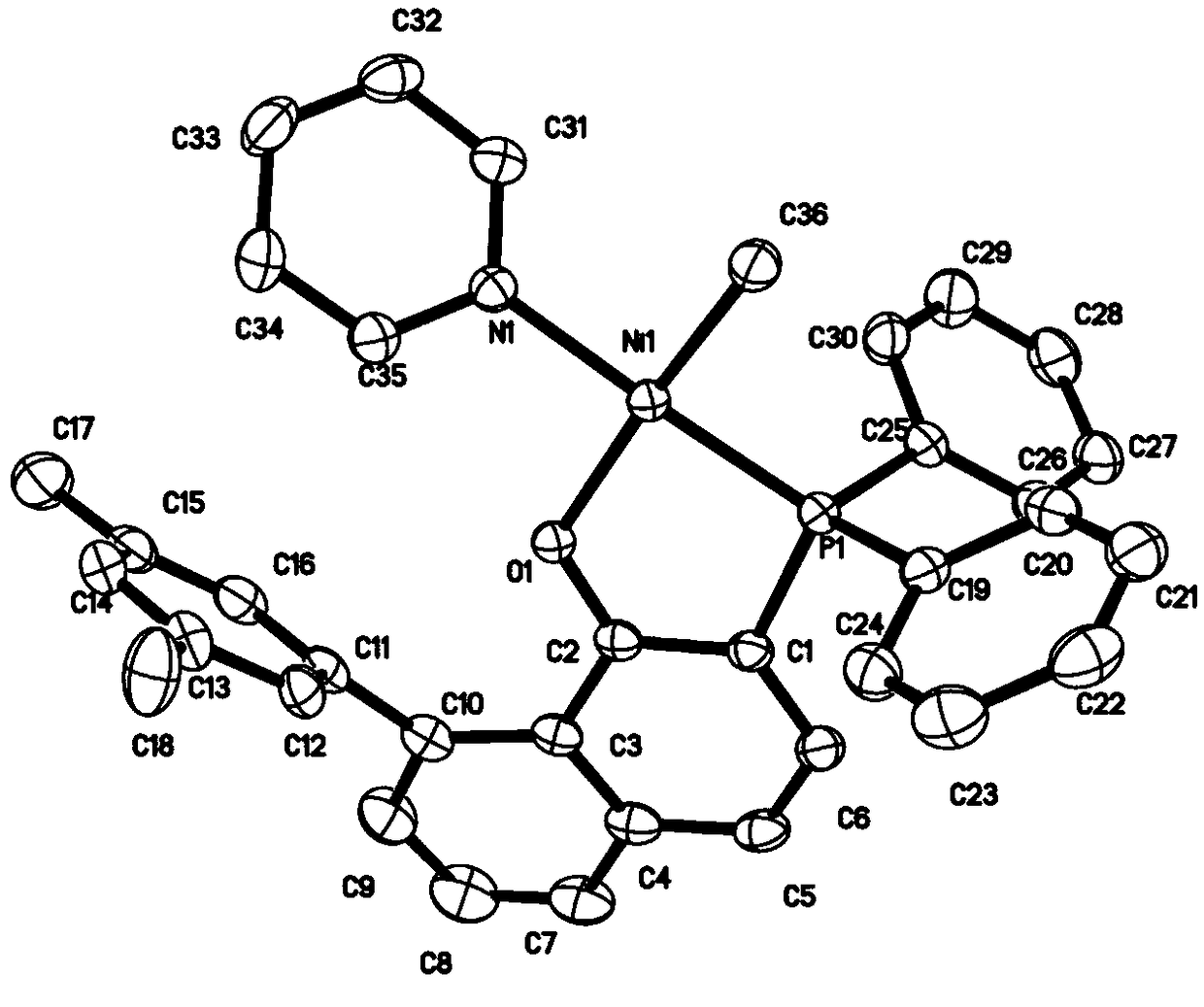
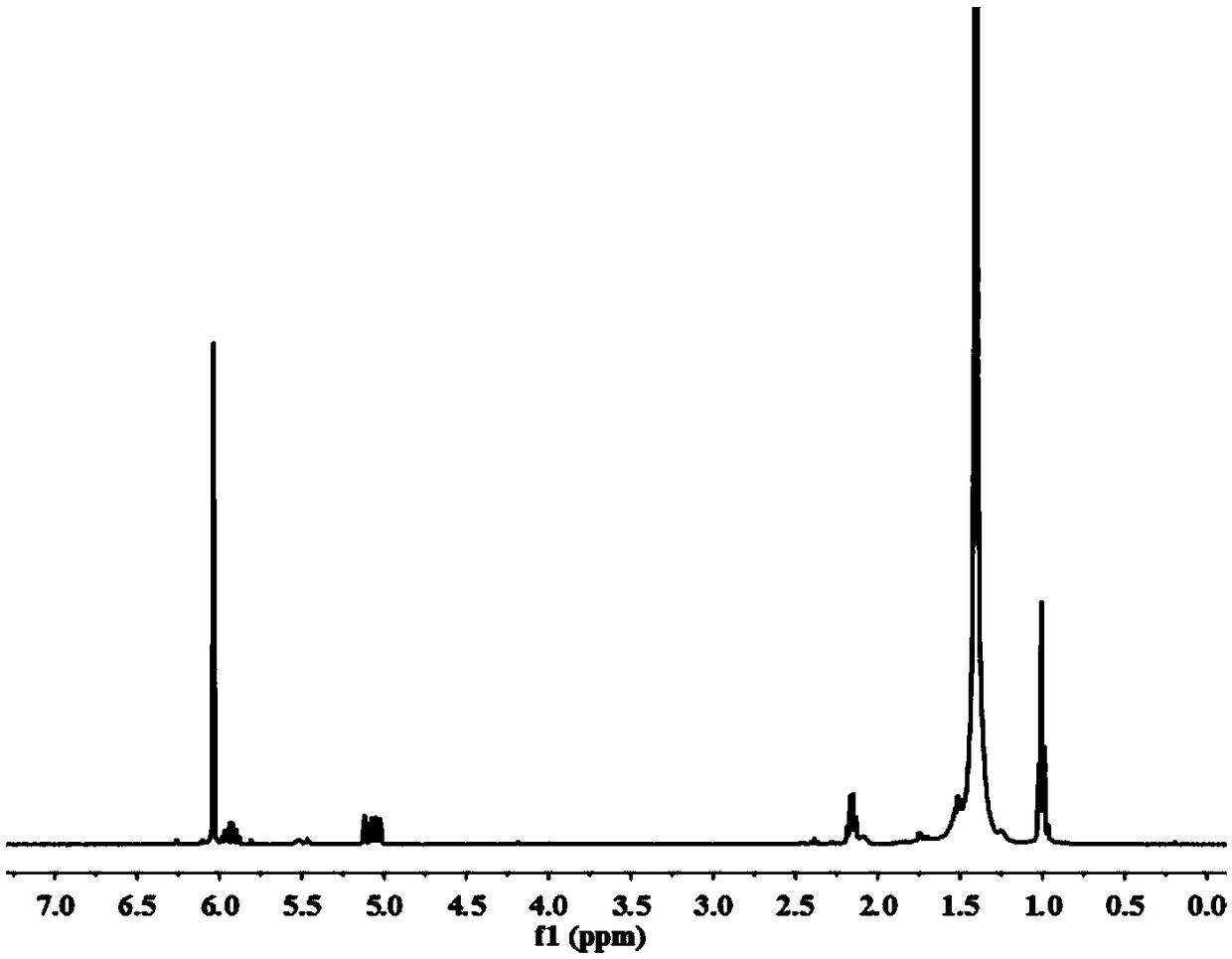
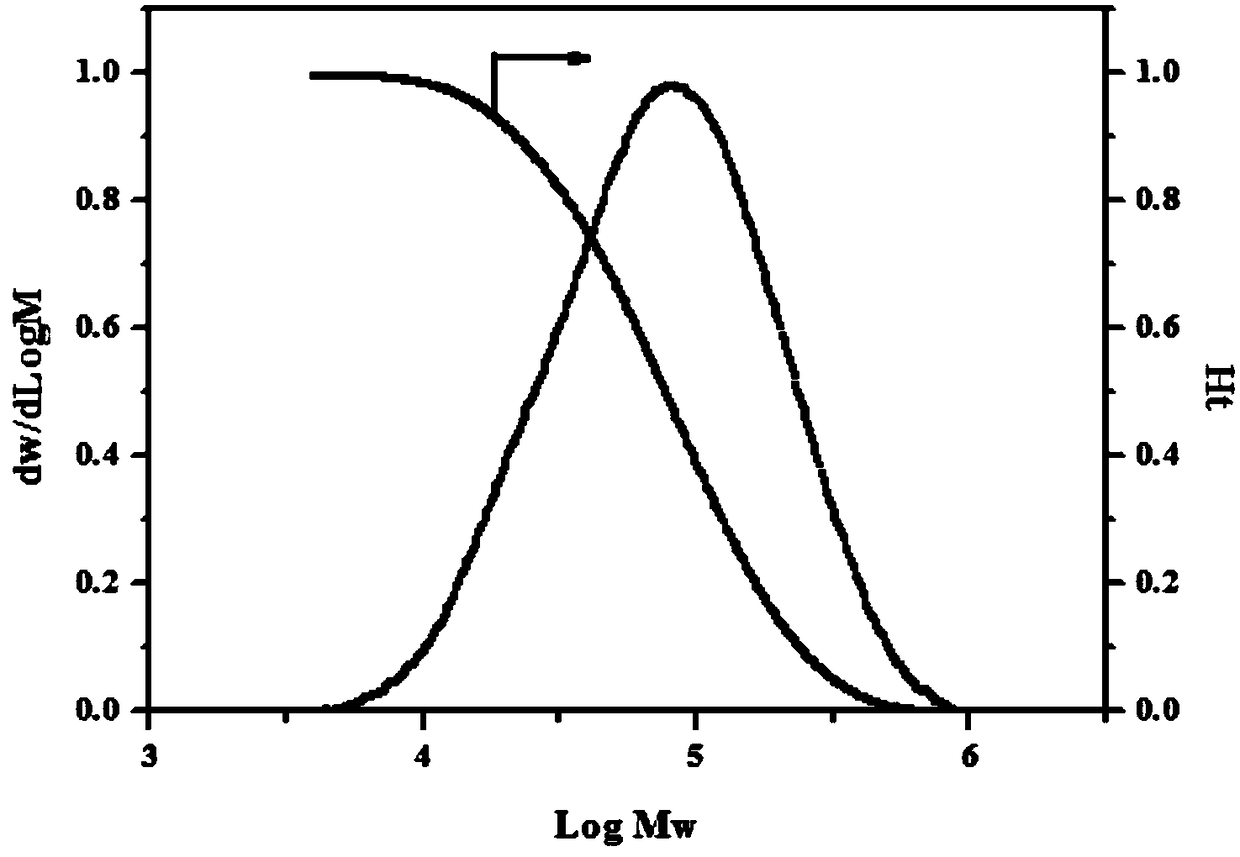
Image
Examples
Embodiment 1
[0046] A-1 ligand is naphthol-phosphine L1, its structural formula is:
[0047]
[0048] Synthetic steps of L1:
[0049] Under a nitrogen atmosphere, α-naphthol (11.5 g, 50 mmol) was reacted with dihydropyran at room temperature for 10 h with stirring to obtain product b with a tetrahydropyran group. It was dissolved in 100 mL of tetrahydrofuran, stabilized at -78°C for 5 minutes, and n-BuLi solution (25 mL, 60 mmol) was added dropwise, and gradually returned to room temperature after 0.5 h of reaction. The reaction was continued for 2h, and a large amount of white precipitate c was obtained. 10.7 mL of diphenylphosphine chloride (PPh 2 Cl) was uniformly dispersed in 20 mL of tetrahydrofuran, and added dropwise to the original reaction system under an ice-water bath, slowly raised to room temperature, and stirred overnight. TLC monitored until the reaction was completed, quenched with water, extracted the organic phase with diethyl ether, concentrated the obtained organi...
Embodiment 2
[0051] A-2 ligand is naphthol-phosphine L2, its structural formula is:
[0052]
[0053] Synthetic steps of L2:
[0054] Under nitrogen atmosphere, dissolve α-naphthol (7.2g, 50mmol), iodobenzene (12.3g, 60mmol), cesium carbonate (19.5g, 60mmol) in 150mL DMF, then add palladium chloride (0.13g, 0.75 mmol) was used as a catalyst, heated to reflux at 115° C. for 12 h, and the reaction progress was monitored by TLC. After the reaction was completed and cooled to room temperature, the organic phase was extracted with ether, and the anhydrous MgSO 4 Dry, filter and concentrate. Column chromatography gave dark brown oily product d. It was stirred and reacted with dihydropyran at room temperature for 10 h to obtain light yellow solid e with tetrahydropyran group.
[0055] Under a nitrogen atmosphere, the intermediate product e (9.2 g, 30 mmol) was dissolved in 50 mL of diethyl ether, stabilized in an ice-water bath for 5 minutes, and n-BuLi solution (14 mL, 33 mmol) was added ...
Embodiment 3
[0057] A-3 ligand is naphthol-phosphine L3, its structural formula is:
[0058]
[0059] Synthetic steps of L3:
[0060] Under nitrogen atmosphere, dissolve α-naphthol (7.2g, 50mmol), 3,5-dimethyliodobenzene (13.9g, 60mmol), cesium carbonate (19.5g, 60mmol) in 150mL DMF, and then add chlorine Palladium chloride (0.13g, 0.75mmol) was used as a catalyst, heated to reflux at 115°C for 12h, and the reaction progress was monitored by TLC. After the reaction was completed and cooled to room temperature, the organic phase was extracted with ether, and the anhydrous MgSO 4 Dry, filter and concentrate. Column chromatography gave dark brown oily product g. It was stirred and reacted with dihydropyran at room temperature for 10 h to obtain light yellow solid h with tetrahydropyran group.
[0061] Under a nitrogen atmosphere, the intermediate product h (9.9 g, 30 mmol) was dissolved in 50 mL of diethyl ether, stabilized in an ice-water bath for 5 minutes, and n-BuLi solution (14 mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com