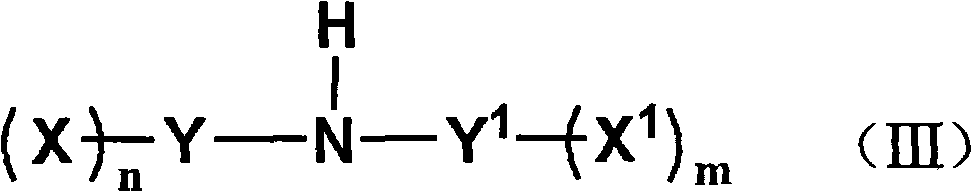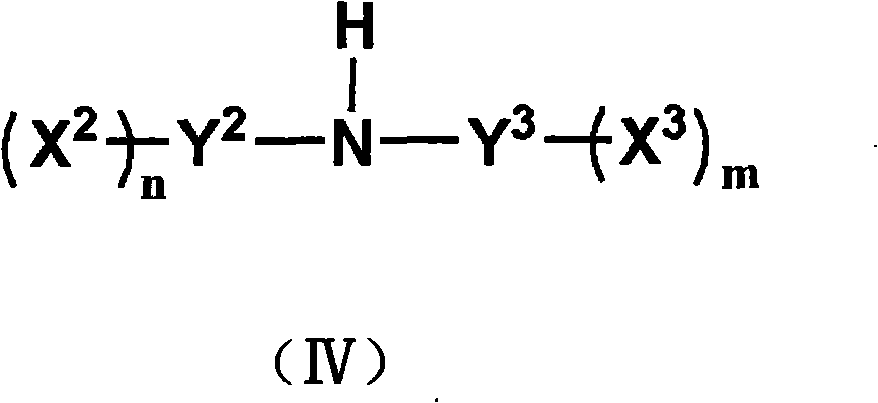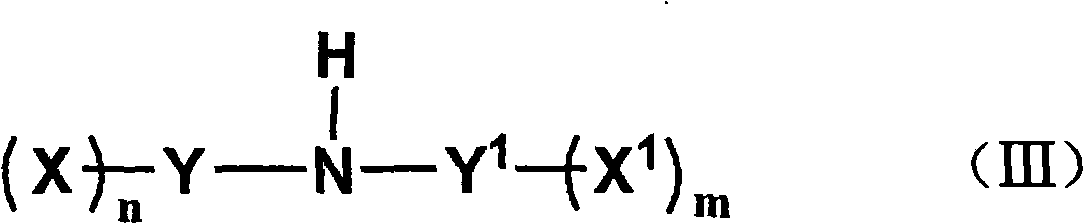Method for preparing fluorine-containing sulphonyl (phosphoryl) imine and alkali metal salt thereof
A technology containing fluorine-containing sulfonyl and perfluoroalkylsulfonyl fluoride, which is applied in the field of fluorine chemical synthesis, and can solve problems such as strong corrosion, harsh reaction conditions, and cumbersome product separation operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the preparation of two (fluorosulfonyl) imides
[0074] The reaction formula is as follows:
[0075]
[0076] To a 500 mL autoclave (PTFE lined) with a reflux condenser, 214 g (1 mol) of bis(chlorosulfonyl)imide (HN(SO 2 Cl) 2 ), 100g anhydrous hydrogen fluoride (HF) (5mol), 8.9g (0.05mol) antimony trifluoride (SbF 3 ) as a catalyst, keep the condenser temperature at -20°C and the heating temperature at 50°C, react for 10 hours, and then stop the reaction. At 50°C, use a dry nitrogen flow to remove volatile components such as excess anhydrous hydrogen fluoride and hydrogen chloride, discharge the material, carry out vacuum distillation, collect the fraction at 82-84°C / 30mmHg, and obtain 159g (0.88mol) of colorless and transparent The liquid product bis(fluorosulfonyl)imide (HN(SO 2 f) 2 ), yield 88%.
Embodiment 13
[0082] Example 13: Preparation of bis(difluorophosphoryl)imide
[0083] The reaction formula is as follows:
[0084]
[0085] Into a 500 mL autoclave (PTFE lined) with a reflux condenser, add 252 g (1 mol) of bis(dichlorophosphoryl)imide (HN(POCl 2 ) 2 ), 160g anhydrous hydrogen fluoride (HF) (8mol), 15g (0.05mol) antimony pentachloride (SbCl 5 ), keep the temperature of the condenser at -20°C, slowly raise the temperature to 50°C and react for 4 hours, then stop the reaction. After removing volatile components such as excess anhydrous hydrogen fluoride and hydrogen chloride with a dry nitrogen flow at 50°C, discharge the material, carry out vacuum distillation, collect 96-100°C / 30mmHg fractions to obtain 148g colorless and transparent bis(difluoro Phosphoryl) imide product, yield 80%.
Embodiment 14
[0086] Example 14: Preparation of (trifluoromethylsulfonyl)(fluorosulfonyl)imide
[0087] The reaction formula is as follows:
[0088]
[0089] To a 500 mL autoclave (PTFE lined) with a reflux condenser, 248 g (1 mol) of (trifluoromethylsulfonyl)(chlorosulfonyl)imide HN(CF 3 SO 2 )(SO 2 Cl), 30 anhydrous hydrogen fluoride (HF) (1.5mol), 4.5g perfluoroheptyl fluoride (C 7 f 15 COF) as a catalyst, keep the temperature of the condenser at -20°C, slowly raise the temperature to 60°C and react for 8h, then stop the reaction. At 50°C, use a dry nitrogen flow to remove volatile components such as excess anhydrous hydrogen fluoride and hydrogen chloride, discharge the material, carry out vacuum distillation, collect 86-90°C / 30mmHg fractions to obtain 162g colorless and transparent (trifluoromethyl Sulfonyl)(fluorosulfonyl)imide liquid, yield 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com