AIDS vaccine of N1L and B8R gene deletion-based vaccinia virus vector
A technology of vaccinia virus and AIDS, applied in virus/bacteriophage, antiviral agent, genetic engineering, etc., can solve serious problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Construction of transfer plasmid
[0053] 1. Construction of transfer plasmid pSKN1L
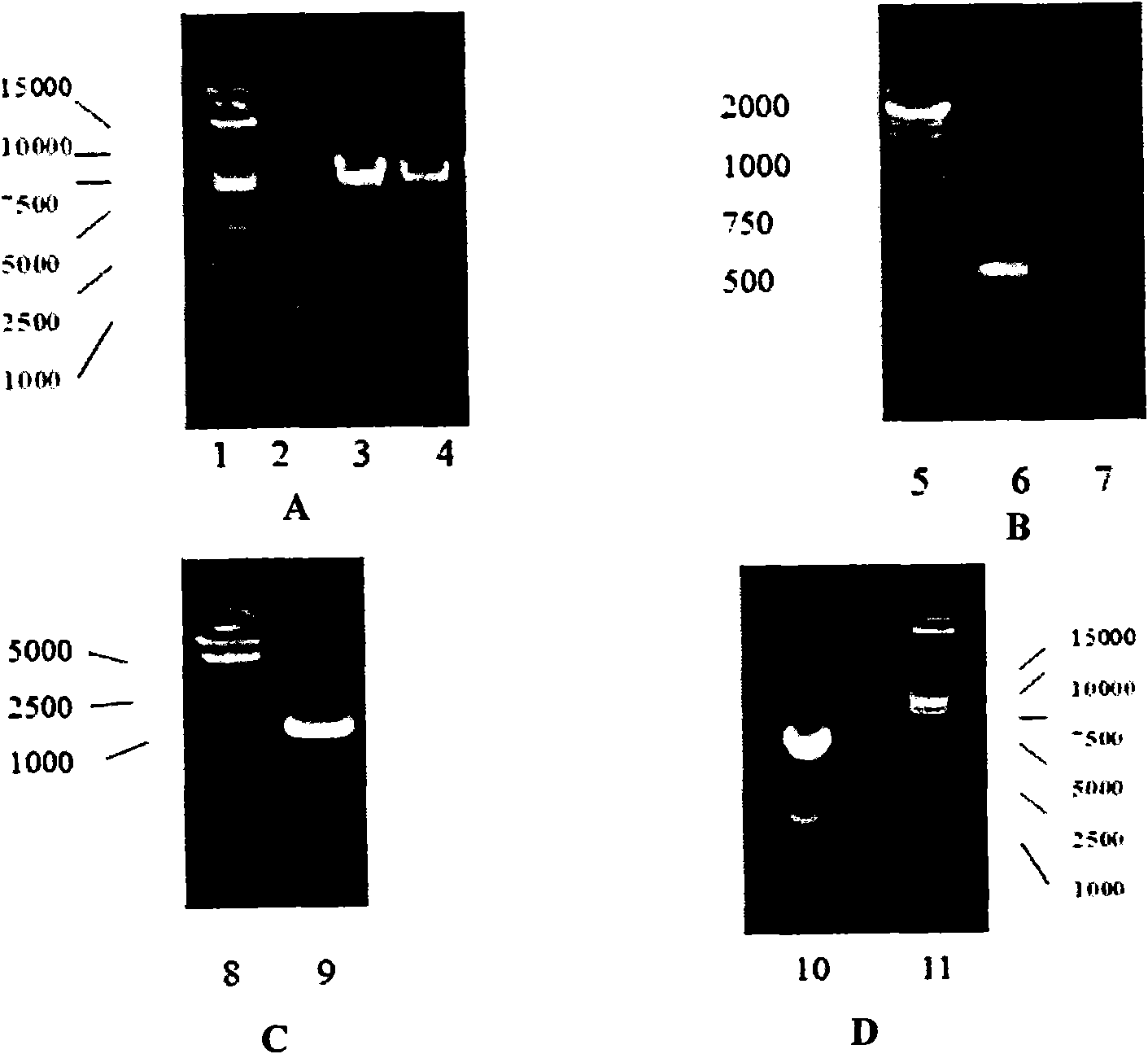
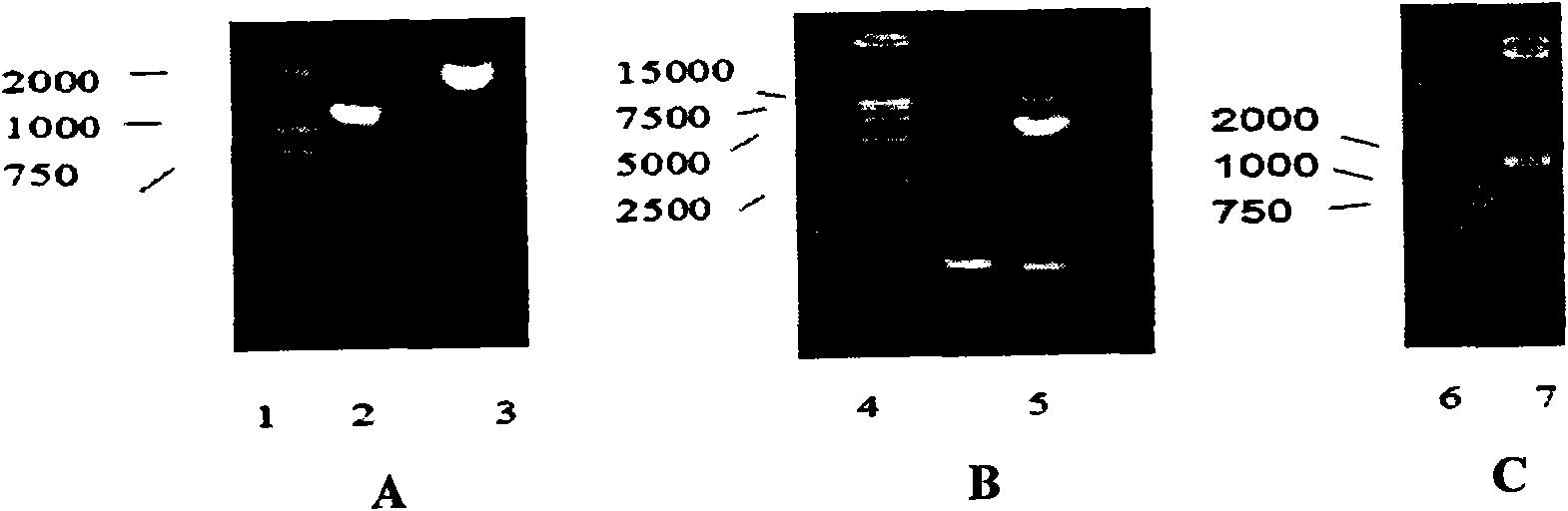
[0054] The DNA of the vaccinia virus Tiantan strain (purchased from Beijing Institute of Biological Products) was used as a template to amplify the left homologous sequence N1LL fragment of the vaccinia virus Tiantan strain N1L gene (primers: SEQID NO.: 1 and 2) and the same The source sequence N1LR fragment (primers: SEQ ID NO.: 3 and 4), the N1LL fragment and the N1LR fragment were respectively digested with Bgl II and ligated, and the ligated product was used as a template for PCR amplification (primers: SEQ ID NO. : 1 and 4) The left and right homologous sequences of the N1L gene are connected into a large fragment, which is called N1LLR. The large fragment N1LLR, vector plasmid pBR-SK (restriction map see Figure 17 ) were co-digested with KpnI / SalI respectively, and after digestion, the two were ligated with T4 DNA ligase to construct a transfer plasmid pSKN1L wit...
Embodiment 2
[0101] Embodiment 2: The construction of the recombinant vaccinia virus inserted HIV-1 gag, pol, env genes of the present invention, and deleted B8R and N1L genes
[0102] 1. Preparation of recombinant vaccinia virus of the present invention by transfection
[0103] Infect 80- 90% chicken embryo fibroblasts (CEF, purchased from Beijing Experimental Animal Center) (MOI=0.01~0.1) in sheets, after 1 hour of adsorption at 37°C, the culture supernatant was discarded and replaced with serum-free Eagle's medium ( The Chinese Center for Disease Control and Prevention (Institute of Viral Disease Prevention and Control) rinsed the cells twice. Using the transfection kit (Lipofectin Reagent Cat. 18292-011) of Invitrogen Company, the above-mentioned CEF cells infected with the recombinant vaccinia virus VTKgpeΔB8R were transfected with the transfer plasmid pSKN1LLacZ according to the instructions of the kit instructions. After the transfection, the transfection mixture was frozen and th...
Embodiment 3
[0139] Example 3: Research on the Impact of Deleting N1L and B8R Genes on the Safety of HIV-1 Tiantan Strain Recombinant Vaccine
[0140] 1. Rabbit skin toxicity test
[0141] Six New Zealand rabbits (purchased from Beijing Kaiyuan Rabbit Industry Farm) (2kg) were shaved on the back, and the back of each rabbit was injected intradermally and simultaneously inoculated with 1×10 6 VTT, VTKgpe, VTKgpeΔB8R, VTKgpeΔB8RΔN1LLacZ of PFU. Observe and record the redness and swelling of the skin injection site for 14 consecutive days, and measure the infiltration diameter and necrosis diameter with a caliper.
[0142] The results showed that the skin acne infiltration toxicity of VTKgpeΔB8RΔN1LLacZ was 100 times lower than that of VTKgpe, and the scar on the skin surface formed by it was significantly smaller than that of VTKgpe and VTKgpeΔB8R (results as follows Figure 8 and Table 1), indicating that deletion of the N1L gene on the basis of VTKgpeΔB8R can significantly reduce the ski...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com