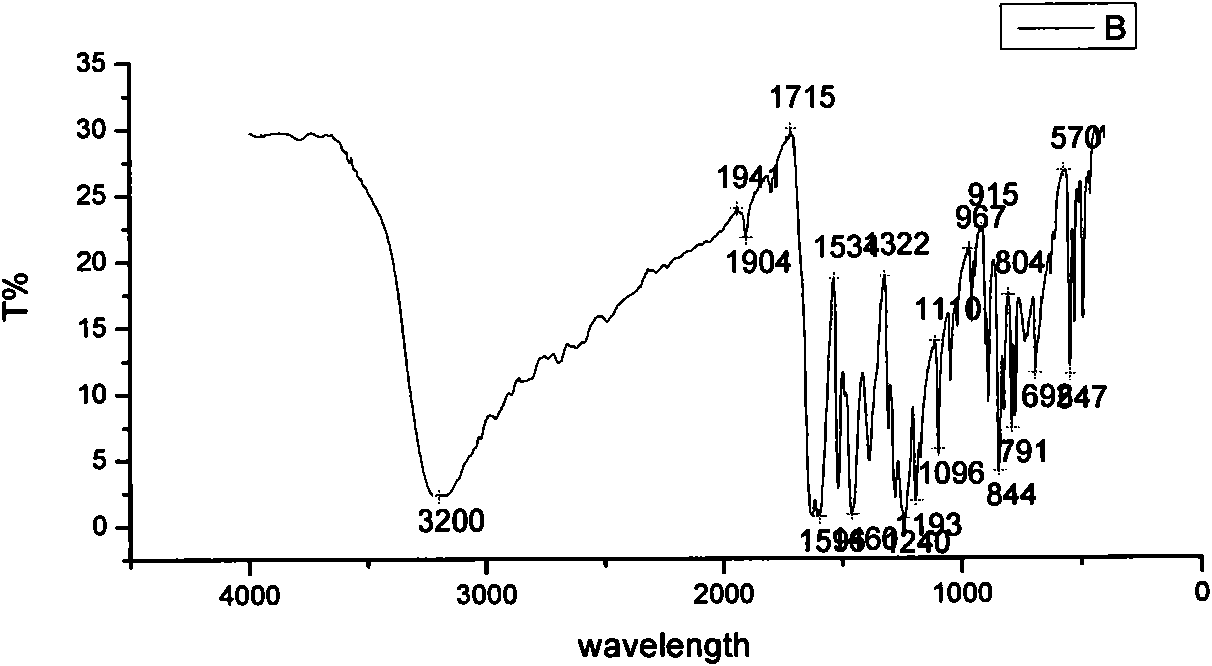
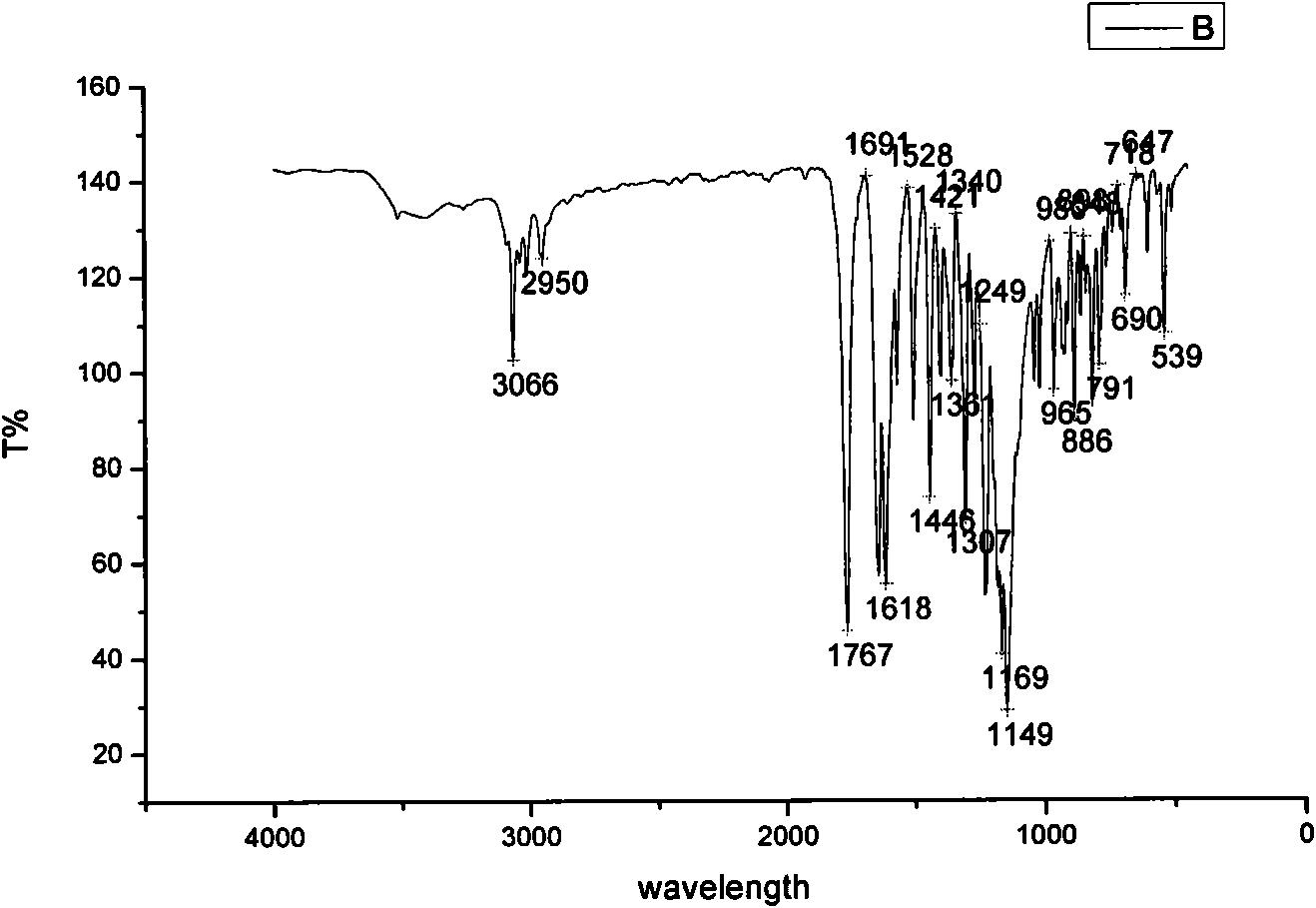
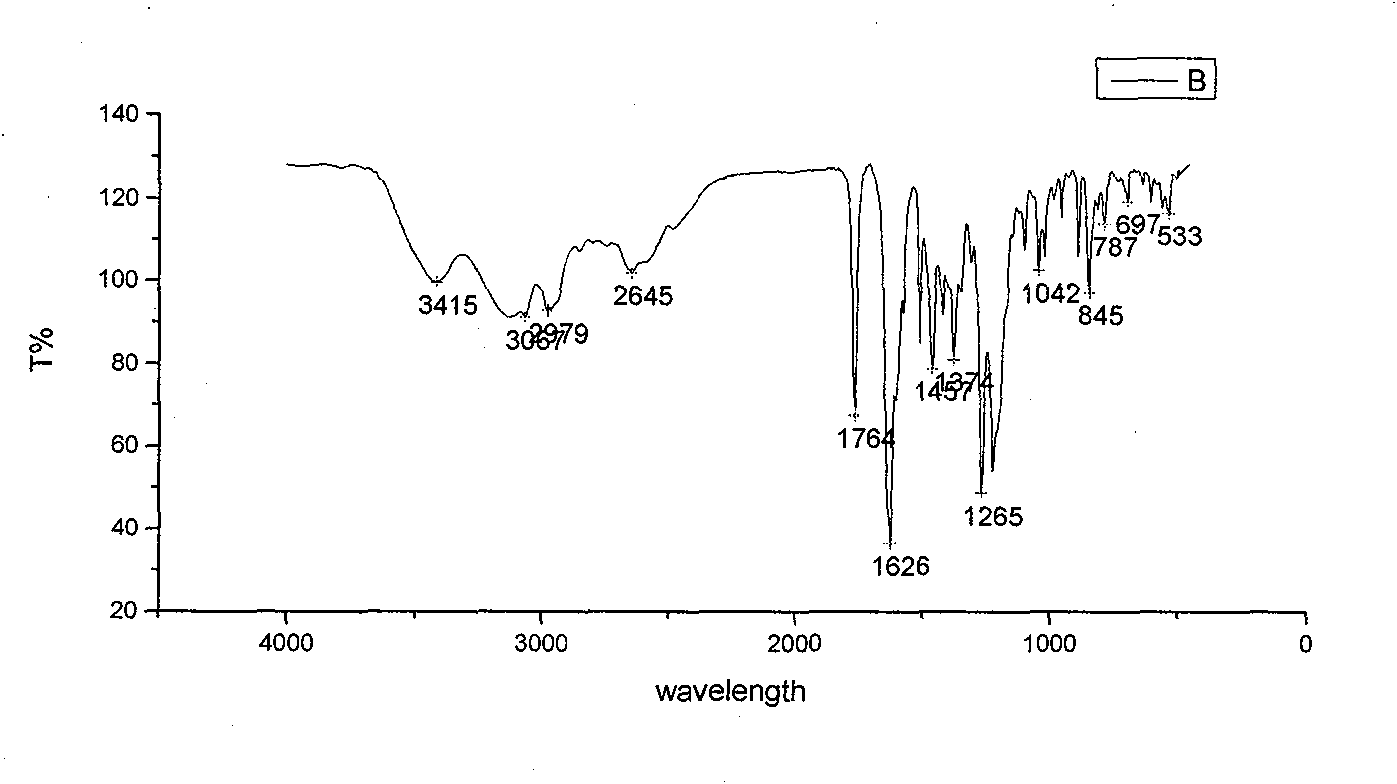
Daidzein derivative and preparation method thereof
A technology of daidzein and its derivatives, applied in the field of modified natural pharmaceutical compounds and their modification, to achieve the effects of improving oral bioavailability, enhancing absorption, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Example 1: Daidzein-7,4'-dioxo-diethylamino-acetate hydrochloride
[0035] The synthetic route is as follows:
[0036]
[0037] The specific operation is as follows:
[0038] (1) Preparation of 7,4′-chloroacetyldaidzein: Take a 500ml dry anhydrous round-bottomed three-neck flask, add 3g daidzein, 150ml ethyl acetate, and 10ml triethylamine, and set up a normal pressure dripping device. The mixture of 10ml of chloroacetyl chloride and 20ml of ethyl acetate was added dropwise in the ice-water bath to 0-3°C, and the dropping time was controlled within 40min. The reaction progress was monitored by TLC, and the reaction was carried out in the ice-water bath for 1.5 hours. Remove the water bath, continue to react at room temperature for 2 hours, filter with suction, and wash the obtained solid with water until neutral. The obtained solid was separated by a silica gel column, and the eluent was ethyl acetate:petroleum ether=1:1, and white powdery solid intermediate I' ...
Embodiment 2
[0040] 2. Example 2: Daidzein-4'-oxygen-diethylamino-acetate hydrochloride
[0041] The synthetic route is as follows:
[0042]
[0043] The specific operation is as follows:
[0044] (1) Preparation of 4′-chloroacetyl daidzein: Take a 500ml dry anhydrous round-bottomed three-neck flask, add 3g daidzein, 150ml ethyl acetate, and 5ml triethylamine, and set up a normal pressure dripping device. The mixture of 5ml of chloroacetyl chloride and 10ml of ethyl acetate was added dropwise in the ice-water bath to 0-3°C, and the dropping time was controlled within 40 minutes. The reaction progress was monitored by TLC, and the reaction was carried out in the ice-water bath for 1.5 hours. Remove the water bath, continue to react at room temperature for 2 hours, filter with suction, and wash the obtained solid with water until neutral. The obtained solid was separated by a silica gel column, and the eluent was ethyl acetate:petroleum ether=1:1, and intermediate I was obtained as a wh...
Embodiment 3
[0051] 3. Example 3: Daidzein 7,4'-oxygen-aminoguanidine-acetate
[0052] The synthetic route is as follows:
[0053]
[0054] The specific operation is as follows:
[0055] (1) Preparation of 7,4′-chloroacetyldaidzein: Take a 500ml dry anhydrous round-bottomed three-neck flask, add 3g daidzein, 150ml ethyl acetate, and 10ml triethylamine, and set up a normal pressure dripping device. The mixture of 10ml of chloroacetyl chloride and 20ml of ethyl acetate was added dropwise in the ice-water bath to 0-3°C, and the dropping time was controlled within 40min. The reaction progress was monitored by TLC, and the reaction was carried out in the ice-water bath for 1.5 hours. Remove the water bath, continue to react at room temperature for 2 hours, filter with suction, and wash the obtained solid with water until neutral. The obtained solid was separated by a silica gel column, and the eluent was ethyl acetate:petroleum ether=1:1, and brown powdery solid intermediate I' was obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com