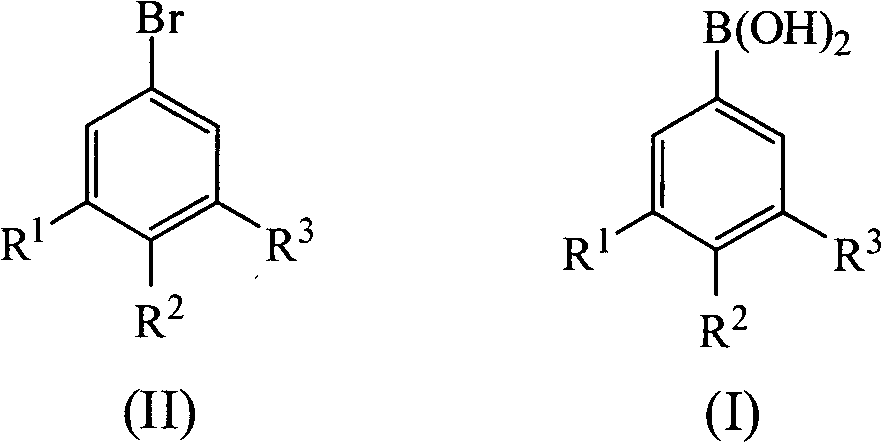
Method for preparing fluoride-bearing phenyloboric acid
A technology of fluorine-containing benzene boronic acid, bromobenzene: magnesium: borate ester, applied in the field of preparation of fluorine-containing benzene boronic acid, can solve the problems of harsh conditions, large operation energy consumption, large operation danger, etc., and achieves low water solubility, The effect of good purity and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The molar ratio of 3-fluorobromobenzene:magnesium:trimethyl borate is 1.0:1.1:1.5. The total mass amount of the solvent 2-methyltetrahydrofuran is 7 times the mass of 3-fluorobromobenzene.
[0040] Add magnesium chips 2.6g (0.11mol) and 1-2 grains of iodine, 2-methyltetrahydrofuran 30g, N 2Protected at 40°C, a small amount of 17.5 g (0.10 mol) of 3-fluorobromobenzene in 22.5 g of 2-methyltetrahydrofuran solution was added dropwise, and after stirring to initiate the reaction, the remaining 3-fluorobromobenzene solution was added dropwise. After the dropwise addition, react at this temperature for 3 hours to obtain a solution of 3-fluorophenylmagnesium bromide in 2-methyltetrahydrofuran.
[0041] Take another 500mL four-necked bottle, add trimethyl borate 15.6g (0.153mol) and 2-tetrahydrofuran 90.0g, N 2 Cool to -20°C under protection, add the above-mentioned Grignard reagent solution dropwise, and keep warm for 3 hours after the dropwise addition. At this temperature...
Embodiment 2
[0049] The molar ratio of 3-fluorobromobenzene:magnesium:trimethyl borate is 1.0:1.05:1.5. The total mass amount of the solvent 2-methyltetrahydrofuran is 7 times the mass of 3-fluorobromobenzene.
[0050] Add 2.5g (0.105mol) of magnesium chips and 1 to 2 grains of iodine, 18.5g of 2-methyltetrahydrofuran, and N 2 Protected at 45°C, a small amount of 17.5 g (0.10 mol) of 3-fluorobromobenzene in 52 g of 2-methyltetrahydrofuran solution was added dropwise, and after stirring to initiate the reaction, the remaining 3-fluorobromobenzene solution was added dropwise. After the dropwise addition, react at this temperature for 2.5 hours to obtain a solution of 3-fluorophenylmagnesium bromide in 2-methyltetrahydrofuran.
[0051] Take another 500mL four-neck bottle, add 15.6g (0.15mol) of trimethyl borate and 52.0g of 2-methyltetrahydrofuran, N 2 Cool to -35°C under protection, add the above-mentioned Grignard reagent solution dropwise, and keep warm for 3 hours after the dropwise add...
Embodiment 3
[0053] The molar ratio of 3-fluorobromobenzene:magnesium:trimethyl borate is 1.0:1.1:1.5. The total mass amount of the solvent 2-methyltetrahydrofuran is 6 times the mass of 3-fluorobromobenzene.
[0054] Add magnesium chips 2.6g (0.11mol) and 1-2 grains of iodine, 18g 2-methyltetrahydrofuran, N 2 At 50°C, a small amount of 17.5 g (0.10 mol) of 3-fluorobromobenzene and 43 g of 2-methyltetrahydrofuran solution was added dropwise, and after stirring to initiate the reaction, the remaining 3-fluorobromobenzene solution was added dropwise. After the dropwise addition, react at this temperature for 4 hours to obtain a solution of 3-fluorophenylmagnesium bromide in 2-methyltetrahydrofuran.
[0055] Take another 500mL four-neck bottle, add trimethyl borate 15.6g (0.15mol) and 2-methyltetrahydrofuran 44g, N 2 Cool to -15°C under protection, add the above-mentioned Grignard reagent solution dropwise, and keep warm for 2 hours after the dropwise addition. At this temperature, add 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com