Method for detecting expression quantity of carcinoembryonic antigen mRNA and special kit thereof
A carcinoembryonic antigen and kit technology, applied in biochemical equipment and methods, microbiological determination/inspection, etc., can solve the problems of low positive rate, lack of detection technology, and incapable of quantitative analysis, etc., to achieve accuracy and specificity Good, high-sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
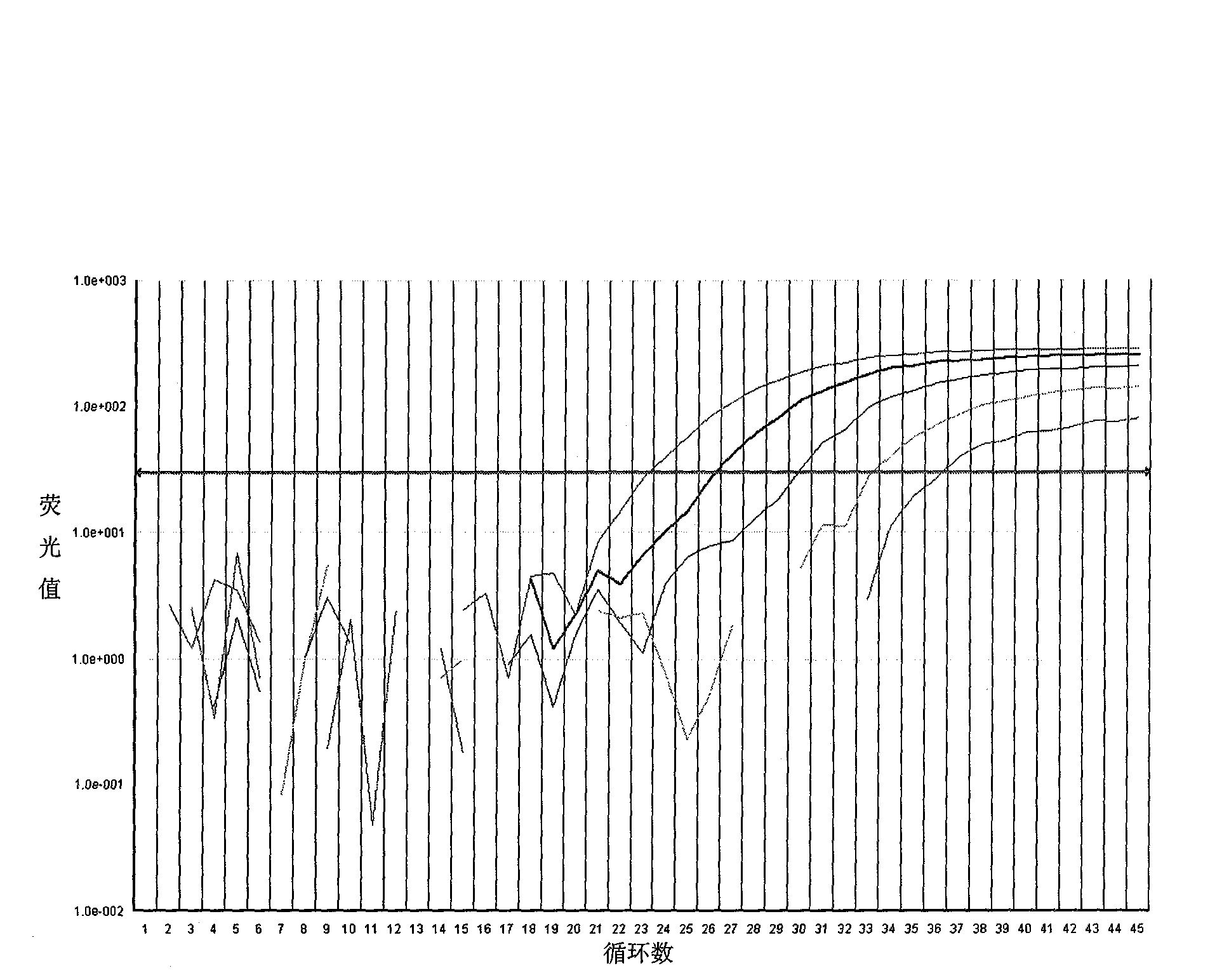
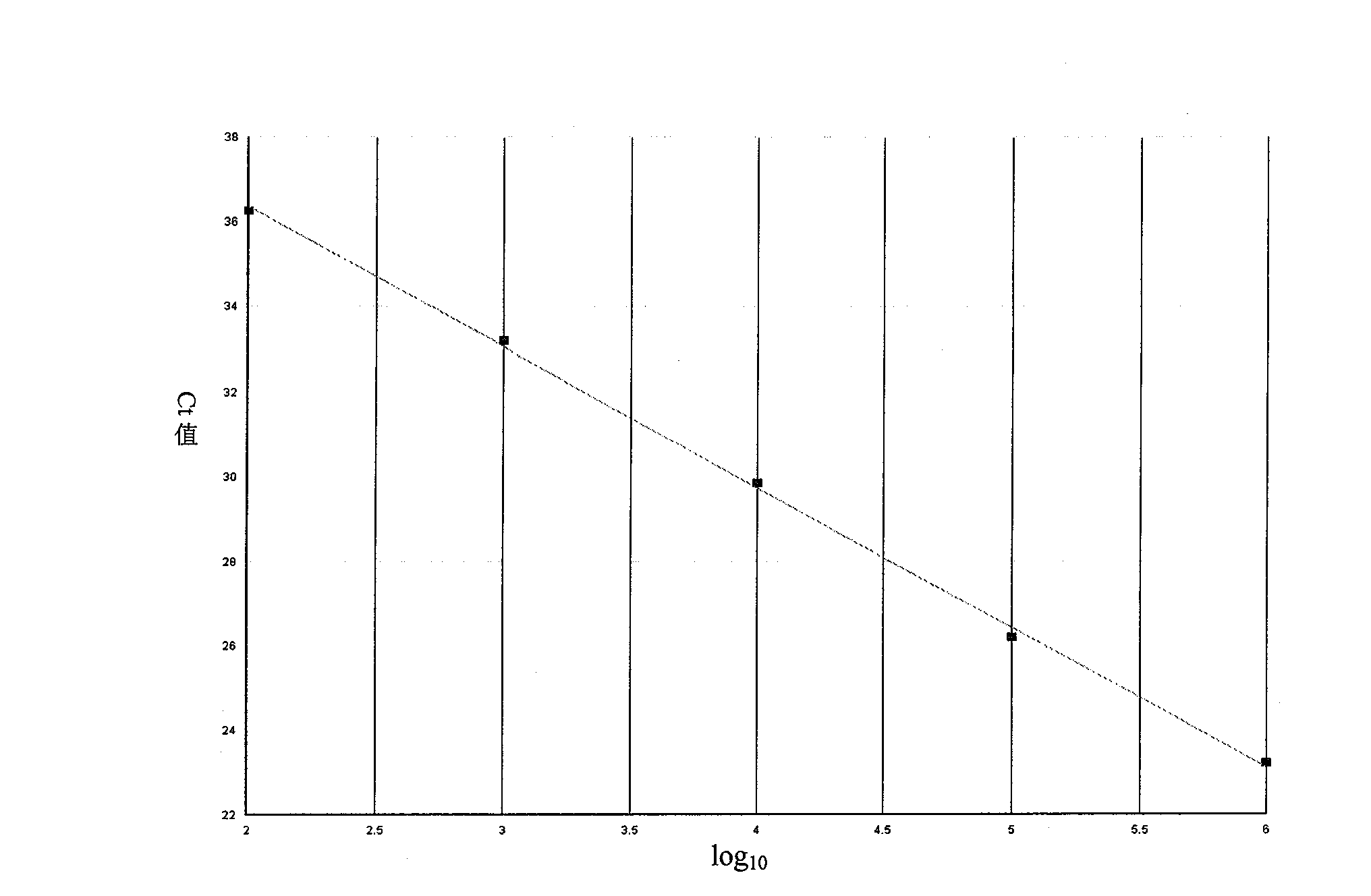
Image
Examples
Embodiment 1
[0046] Embodiment 1, composition and preparation of kit
[0047] The composition of the kit and its preparation method are as follows:
[0048] 1. Cell lysate I
[0049] It consists of tris(hydroxymethyl)aminomethane hydrochloride with a final concentration of 0.1mol / L, sodium chloride with a final concentration of 0.1mol / L and magnesium chloride with a final concentration of 0.05mol / L; the solvent is water. Wherein, the final concentration is the concentration of each substance in cell lysate I.
[0050] Cell lysate I has a pH value of 7.6 at 25°C.
[0051] 2. Cell Lysis Solution II
[0052] It consists of guanidine isothiocyanate with a final concentration of 3mol / L, guanidine hydrochloride with a final concentration of 2mol / L, sodium acetate with a final concentration of 0.3mol / L and sodium lauryl sulfate with a final concentration of 0.2%. It is water. Wherein, the final concentration is the concentration of each substance in the cell lysate II.
[0053] The cell lys...
Embodiment 2
[0182] Embodiment 2, detection of carcinoembryonic antigen gene mRNA expression
[0183] The kit prepared in Example 1 was used to detect the expression of carcinoembryonic antigen gene mRNA in the specimens of the following experimental group and control group.
[0184] Experimental group: 15 patients with pathologically diagnosed gastric cancer, 5 of whom were clinically diagnosed with metastasis; 10 patients with pathologically diagnosed esophageal cancer, 4 of whom were clinically diagnosed with metastasis; 8 patients with pathologically diagnosed colon cancer, Three of them were clinically confirmed to have metastases.
[0185] Control group: 10 patients with colon polyps, 10 patients with gastric ulcer and 10 healthy people.
[0186] All samples were obtained with the consent of the subjects.
[0187] 1. Experiment preparation
[0188] 1. Dilute the cell lysate I with sterilized deionized water at a ratio of 1:9 to obtain the cell lysate I dilution.
[0189] 2. Add 2...
Embodiment 3
[0239] Embodiment 3, test kit and application thereof
[0240] 1. Kit composition and preparation method
[0241] Same as described in Example 1, the difference is as follows:
[0242] 1. PCR reaction buffer: composed of tris(hydroxymethyl)aminomethane hydrochloride with a final concentration of 18.0mmol / L, magnesium chloride with a final concentration of 2.60mmol / L and potassium chloride with a final concentration of 90.5mmol / L , the solvent is water; wherein, the final concentration is the final concentration of each substance in the PCR reaction buffer.
[0243] 2. PCR reaction solution: consisting of the PCR reaction buffer, Taq enzyme, dATP, dCTP, dGTP, dTTP, dUTP, the primer pair shown in sequence 1 and 2 in the sequence listing, the probe shown in sequence 3 in the sequence listing and urine Pyrimidine DNA glycosylase, the rest is water;
[0244] The concentration of the PCR reaction buffer in the PCR reaction solution is 0.9 μl / μl;
[0245] The concentration of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com