Double stimuli responsive type poly amino acid-based supermolecular reverse gel and method for preparing same
A dual-stimuli-response, polyamino acid technology, applied in the field of chemical gel and its preparation, can solve the problems of undiscovered prior art and achieve the effect of simple preparation method and fast gelation speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of linear polyamino acid-based supramolecular reverse gel
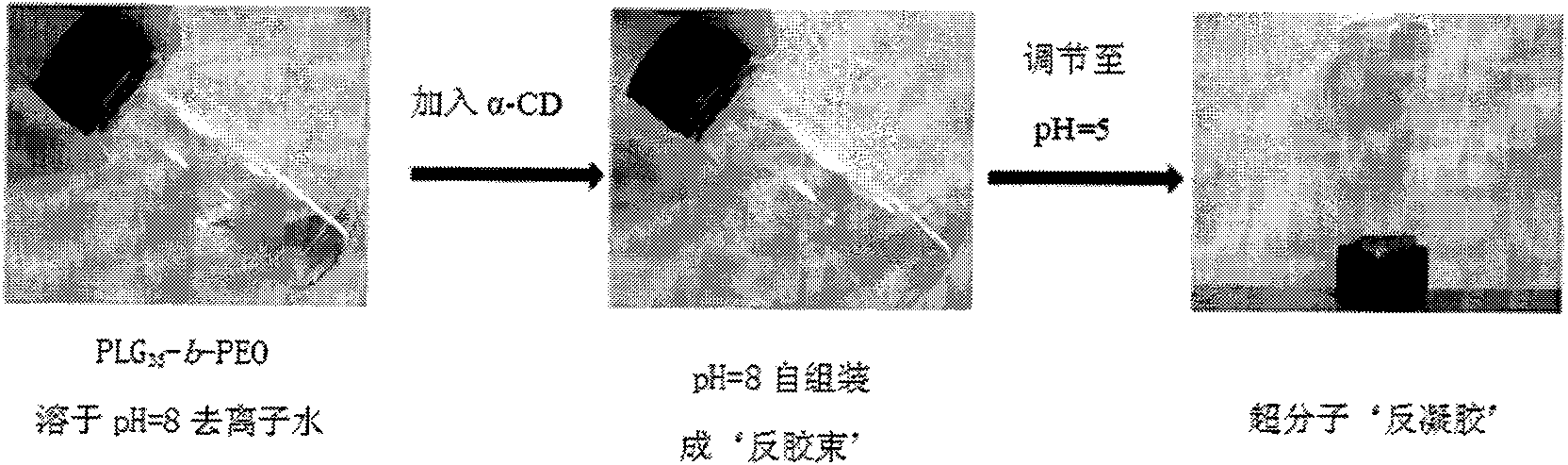
[0024] Polyethylene glycol (MPEO-NH 2 ) as a macroinitiator to initiate the ring-opening polymerization of γ-benzyl-L-glutamic acid-N-carboxy anhydride (BLG-NCA), and then use acetic acid (CH 3 COOH) and hydrobromic acid (HBr) mixed system, after taking off the benzyl group of the PBLG segment, the poly(L-glutamic acid)-polyethylene glycol (PLG) used to form the reverse gel is obtained 35 -b-PEO) diblock copolymer with an arm length of 35 repeating units.
[0025] Using the above diblock copolymer (PLG 35 -b-PEO, 40mg) was added to a 5mL volumetric flask containing magnetic particles, and 1mL deionized water was added. After stirring, the polymer was insoluble, and the solution was cloudy with a small amount of precipitation; 12μL 3mol / L NaOH solution was taken with a micro syringe, and made Its pH ~ 8, at this time the system is clear and transparent, then add 60mg α-CD under vigorous...
Embodiment 2
[0028] Example 2: Preparation of polyamino acid-based supramolecular reverse gel with eight arms
[0029] Using methoxypolyethylene glycol (MPEO-D) with 8 branched amino groups at one end 3 ) as a macroinitiator to initiate the ring-opening polymerization of γ-benzyl-L-glutamic acid-N-carboxy anhydride (BLG-NCA), and then use acetic acid (CH 3 COOH) and hydrobromic acid (HBr) system, after removing the benzyl protection of the PBLG segment, the eight-armed poly(L-glutamic acid)-polyethylene glycol (D3-PBLG) used to form the reverse gel was obtained 13 -b-PEO) a diblock copolymer with an arm length of 13 repeating units.
[0030] Using the above diblock copolymer (D3-PLG 13 -b-PEO, 40mg) was added to a 5mL volumetric flask containing magnetic particles, and 1mL deionized water was added. After stirring, the polymer was insoluble, and the solution was cloudy with a small amount of precipitation; 70μL 3mol / L NaOH solution was taken with a micro-syringe to make Its pH=8, at thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com