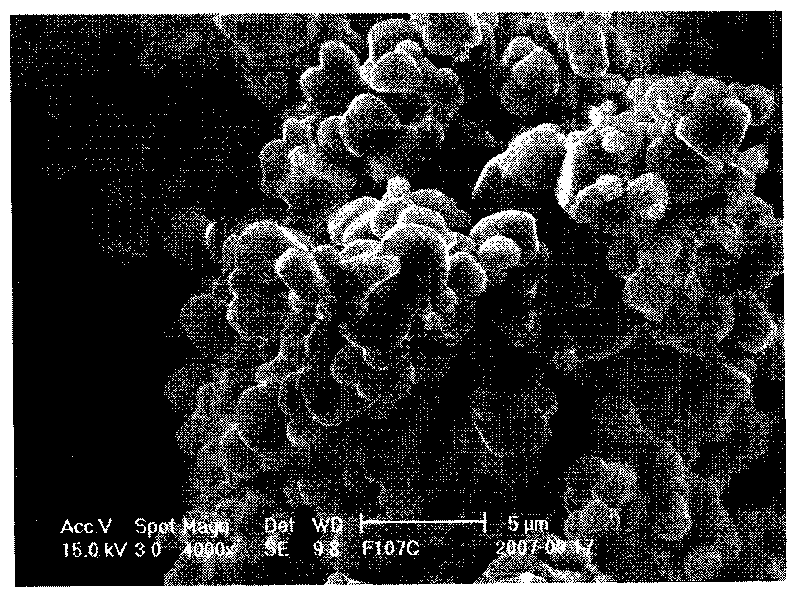Lithium-rich manganese-based anode material and preparation method thereof
A positive electrode material, lithium-rich manganese-based technology, applied in the direction of battery electrodes, electrical components, circuits, etc., can solve the problems of poor cycle stability, material performance that does not meet the requirements of practical applications, and poor material rate performance. Stable performance, excellent rate cycle performance, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
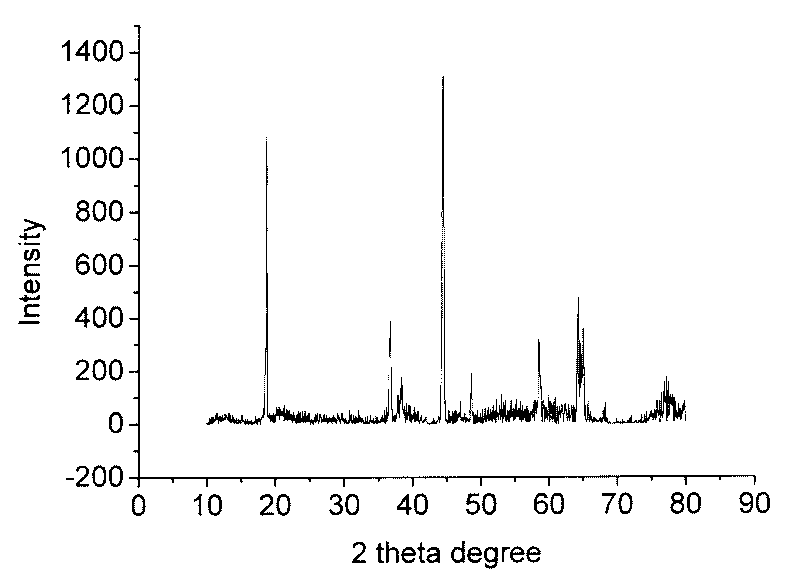
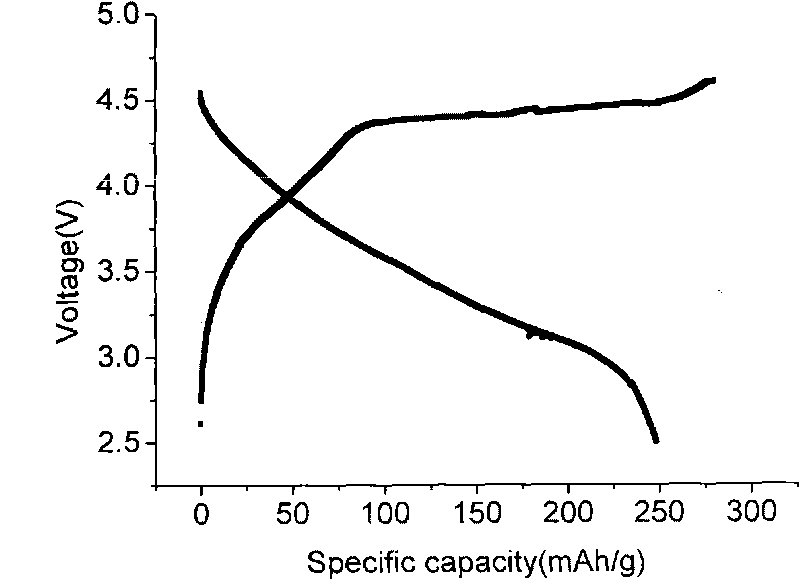
Image
Examples
preparation example Construction
[0043] Lithium-rich manganese-based cathode material Li[Li] provided by the present invention (1-2x) / 3 Ni x-a m y mn (2-x) / 3-b ]O 2 (M=Co, Al, Ti, Mg, Cu) preparation method comprises the following steps:
[0044] a. soluble nickel, manganese, M salt are dissolved in deionized water in a molar ratio of (x-a): [(2-x) / 3-b]: y, and the total concentration is prepared into a solution of 0.5~4mol / L, Wherein, 0<x≤0.5, when M=Co, Al, 0<y<2x, a=b=y / 2; when M=Ti, 0<y<(2-x) / 3, a= 0, b=y; when M=Mg, Cu, 0<y<x, a=y, b=0;
[0045] b. Prepare an alkali solution or a mixed solution of alkali and ammonia water, the alkali concentration is 1-8mol / L, and the molar concentration of ammonia water is 0.1-4mol / L;
[0046] c. add a certain amount of deionized water in the reactor, pump the nickel, manganese, M salt solution into the reactor, and pump the alkali solution into the reactor simultaneously, Stir the materials, control the temperature and pH value in the reactor, and form precursor...
Embodiment 1
[0051]The molar percentage of nickel, manganese and aluminum metal ions is 0.123:0.850:0.027. Dissolve nickel sulfate, manganese sulfate and aluminum sulfate in deionized water to prepare a uniform and transparent solution with a total concentration of manganese, nickel and aluminum ions of 2mol / L , prepare a 4mol / L sodium hydroxide solution, pump the mixed metal salt solution and the sodium hydroxide solution into the reactor at the same time for co-precipitation reaction. The precipitated product was filtered, washed, and dried to obtain the metal hydroxide precursor Mn 0.85 Ni 0.123 Al 0.027 (OH) 2 .
[0052] The precursor and lithium carbonate are uniformly mixed according to the ratio of the molar number of lithium to the total molar number of nickel, manganese, and aluminum of 1.77:1, and the uniformly mixed powder is compacted and kept at 450°C for 2 hours. Then the temperature was raised to 800°C for 40 hours to obtain the lithium-rich manganese-based material Li[L...
Embodiment 2
[0055] With the molar percentage of nickel, manganese, and aluminum metal ions as 0.238:0738:0.025, nickel chloride, manganese sulfate, and aluminum chloride are dissolved in deionized water, and the total concentration of manganese, nickel, and aluminum ions is 2mol / L. Uniform and transparent solution, prepare 4mol / L sodium hydroxide solution, pump the mixed metal salt solution and sodium hydroxide solution into the reactor at the same time for co-precipitation reaction. The precipitated product was filtered, washed, and dried to obtain the metal hydroxide precursor Mn 0.738 Ni 0.238 Al 0.025 (OH) 2 .
[0056] The precursor and lithium carbonate are uniformly mixed according to the ratio of the molar number of lithium to the total molar number of nickel, manganese, and aluminum of 1.54:1, and the uniformly mixed powder is compacted and kept at 550°C for 4 hours. Then the temperature was raised to 900°C for 20 hours to obtain the lithium-rich manganese-based material Li[Li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com