A kind of nickel cobalt lithium manganate modified material and its preparation method and application
A technology of nickel-cobalt lithium manganese oxide and modified materials, which is applied in the field of nickel-cobalt lithium manganese oxide modified materials and its preparation, can solve the problems of limited level and lower energy density of nickel-cobalt lithium manganese oxide, and improve the stable phase, Good cycle performance and the effect of improving energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
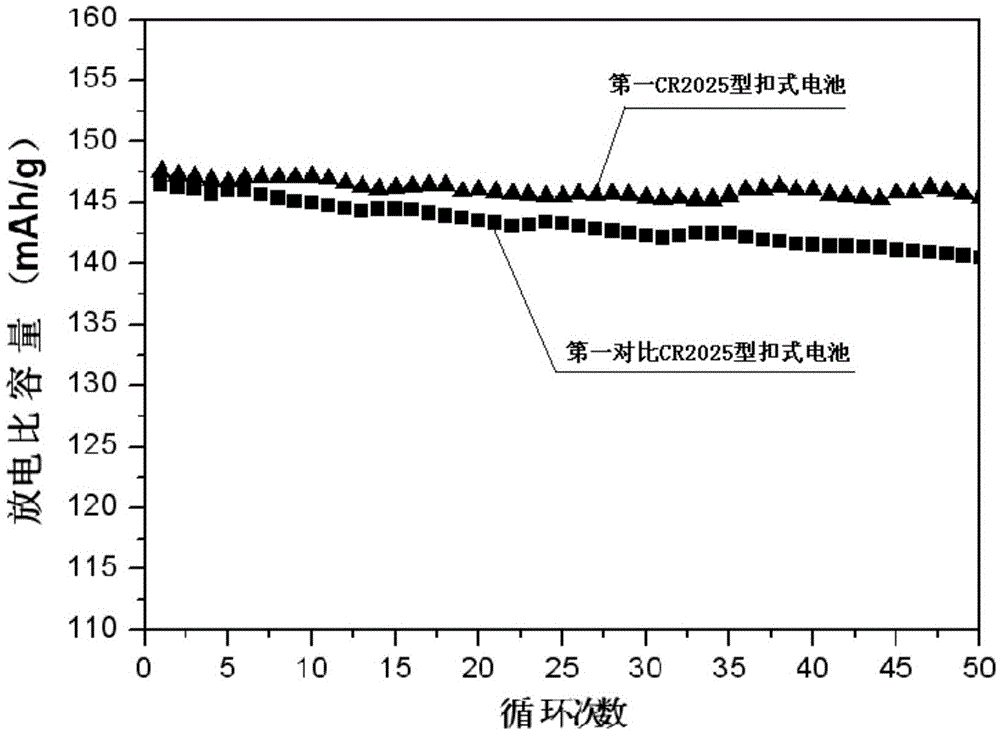
Examples
preparation example Construction
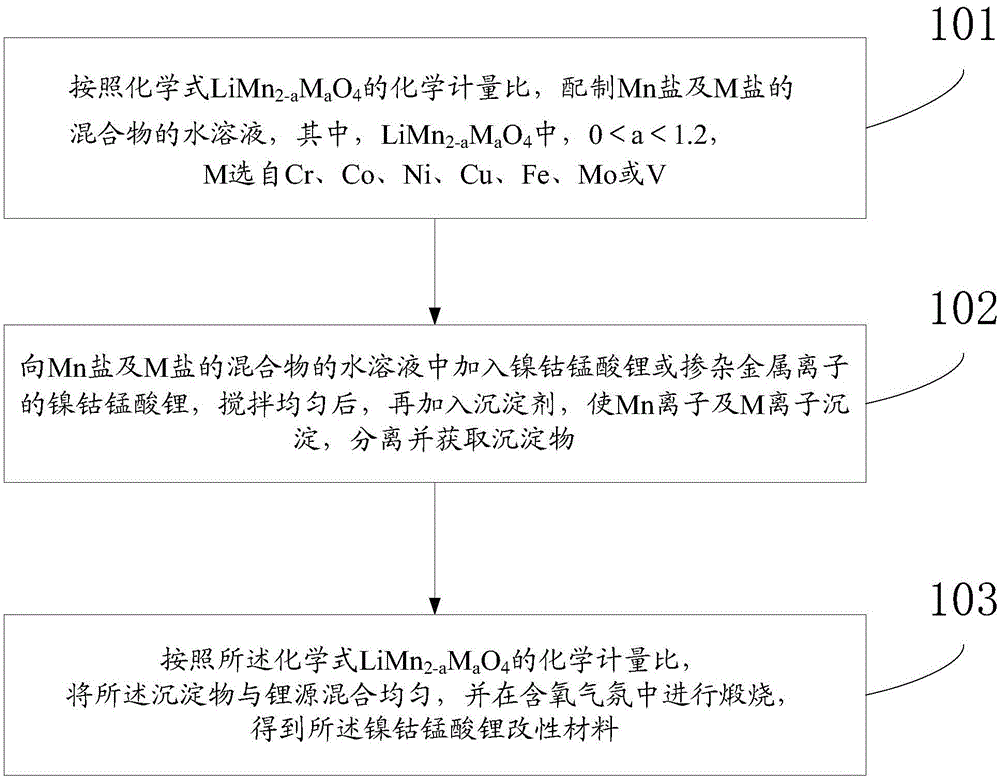
[0049] In the third aspect, the embodiment of the present invention also provides a preparation method of nickel cobalt lithium manganese oxide modified material, with figure 1 For the preparation flow chart of this method, as attached figure 1 As shown, the method includes:
[0050] Step 101, according to the chemical formula LiMn 2-a m a o 4 The stoichiometric ratio, the aqueous solution of the mixture of preparation Mn salt and M salt,
[0051] The LiMn 2-a m a o 4 where 0
[0052] Step 102: Add nickel-cobalt lithium manganate or nickel-cobalt lithium manganate doped with metal ions to the aqueous solution of the mixture of Mn salt and M salt, stir evenly, and then add a precipitant to precipitate Mn ions and M ions , separated and obtained the precipitate.
[0053] Step 103, according to the chemical formula LiMn 2-a m a o 4 The stoichiometric ratio is uniformly mixed with the precipitate and the lithium so...
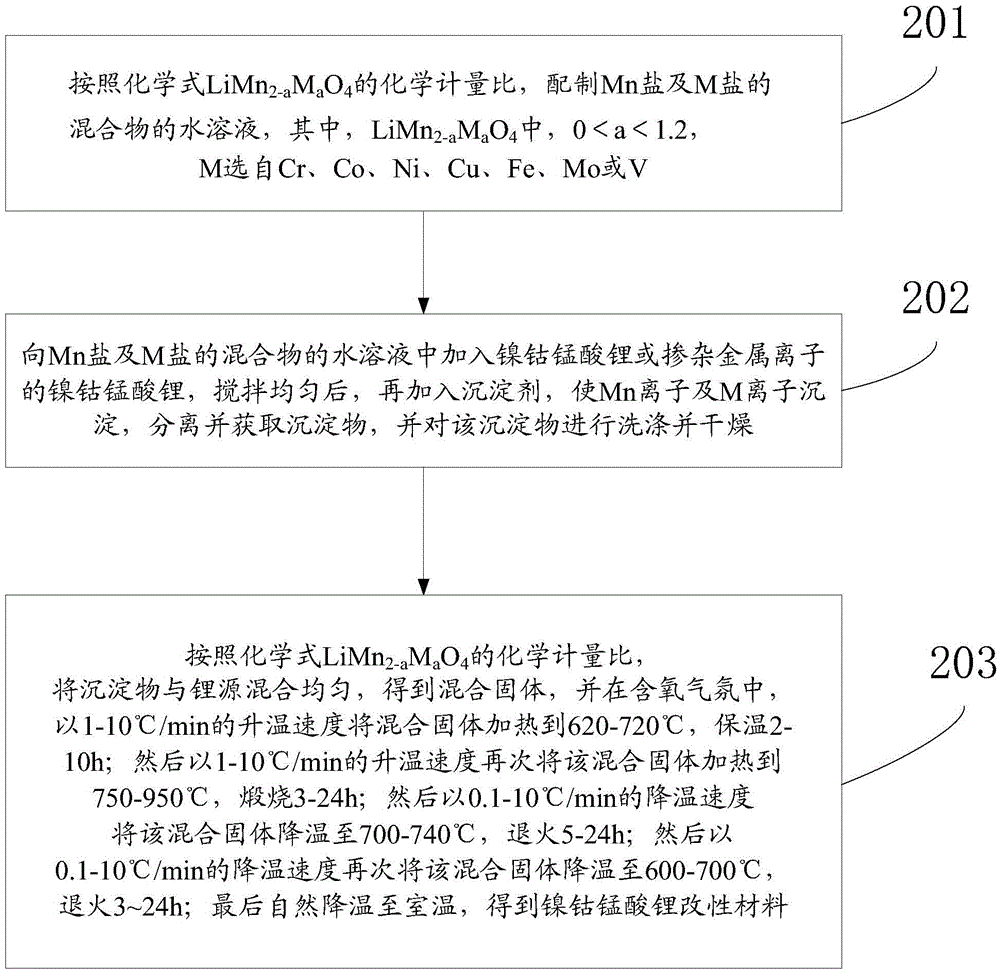
Embodiment 1
[0071] This embodiment provides a LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 Modified materials, including: LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 , and coated in LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 Surface LiNi 0.5 mn 1.5 o 4 , where LiNi 0.5 mn 1.5 o 4 The mass of the LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 2% of the mass of the modified material. The LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 The preparation steps of the modified material are as follows:
[0072] Prepare an aqueous solution of a mixture of soluble nickel chloride and manganese sulfate according to the molar ratio Ni:Mn=1:3, and add LiNi to the aqueous solution of the mixture under stirring 1 / 3 co 1 / 3 mn 1 / 3 o 2 , disperse evenly, and then add sodium carbonate dropwise to the mixed solution to completely precipitate the nickel ions and manganese ions, obtain the precipitate by suction filtration, wash the precipitate 3 times, and dry it.
[0073] The above-mentioned dry precipitate and lithium carbonate (Li excess 2%) are mixed uniformly, then ...
Embodiment 2
[0075] This embodiment provides a LiCoO 2 Modified materials, including: LiCoO 2 , and coated in LiCoO 2 Surface LiFe 0.5 mn 1.5 o 4 , where LiFe 0.5 mn 1.5 o 4 The mass of the LiCoO 2 0.2% of the mass of the modified material. The LiCoO 2 The preparation steps of the modified material are as follows:
[0076] Prepare an aqueous solution of a mixture of soluble ferric chloride and manganese sulfate according to the molar ratio Ni:Mn=1:3, and add LiCoO to the aqueous solution of the mixture under stirring 2 , disperse evenly, and then add dropwise oxalic acid to the mixed solution to completely precipitate the iron ions and manganese ions, obtain the precipitate by suction filtration, wash the precipitate 3 times, and dry it.
[0077] Mix the above-mentioned dried precipitate with lithium acetate (5% excess Li), then program temperature control, and calcinate the mixture of the precipitate and lithium acetate in air. The specific calcination process is as follows: he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com