Chitosan entrapment body of nano-scale arsenous acid and manufacturing method thereof
A nano-chitosan and nano-sized technology, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of high toxicity and side effects, and low bioavailability of arsenic preparations. , to achieve the effect of more active centers, excellent biomedical characteristics, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
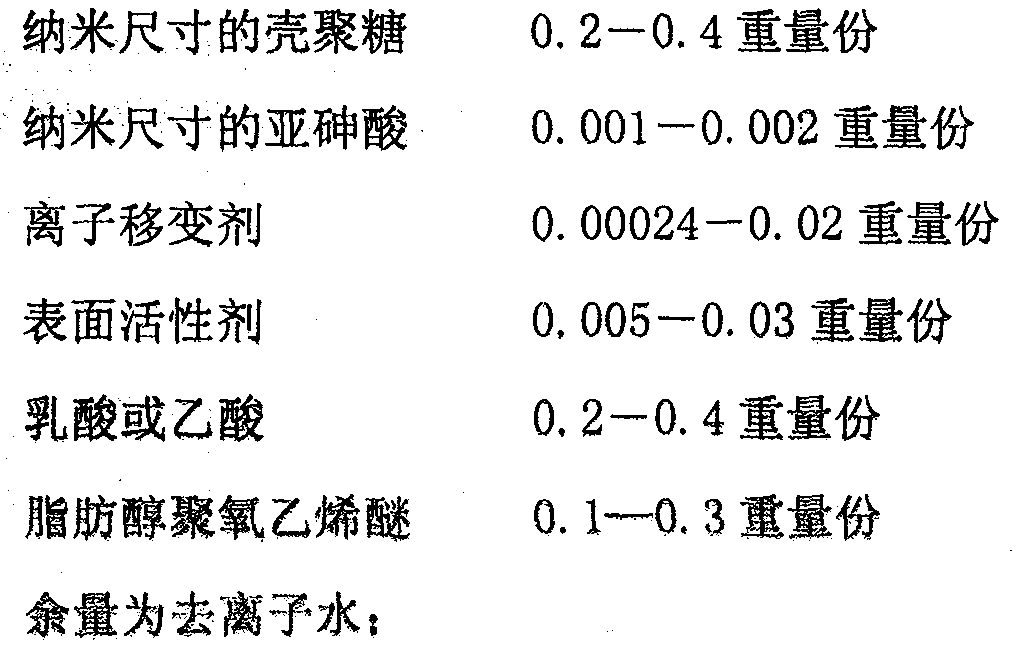
[0039] 1) The chitosan-wrapped carrier of type A nano-sized arsenous acid, by 100 parts by weight, consists of:
[0040] Nano-sized chitosan 0.361 parts by weight
[0041] Nano-sized arsenous acid 0.0018 parts by weight
[0042] Sodium sulfate (ionotrope) 0.0024 parts by weight
[0043] Span-80 (surfactant) 0.0188 parts by weight
[0044] Acetic acid 0.361 parts by weight
[0045] Fatty alcohol polyoxyethylene ether 0.289 parts by weight
[0046] The balance is 98.966 parts by weight of deionized water
[0047] 2) The manufacture method of the chitosan-wrapped carrier of the A-type nano-sized arsenous acid, the steps are as follows:
[0048] (1) Preparation process of arsenous acid pre-dispersed intermediate:
[0049] 1. Take 0.1 parts by weight of Span-80 (surfactant) and 200 parts by weight of deionized water, place them in a reaction kettle equipped with a backflow condenser, adjust the stirring speed to 2000 rpm, Accompanied by stirring for 30 minutes;
[0050] 2. Th...
Embodiment 2
[0062] 1) The chitosan-wrapped carrier of B-type nano-sized arsenous acid, by 100 parts by weight, consists of:
[0063] Nano-sized chitosan 0.238 parts by weight
[0064] Nano-sized arsenous acid 0.00115 parts by weight
[0065] Sodium tripolyphosphate (ionotrope) 0.00024 parts by weight
[0066] Tween-80 (surfactant) 0.006 parts by weight
[0067] Lactic acid 0.238 parts by weight
[0068] Fatty alcohol polyoxyethylene ether 0.19 parts by weight
[0069] The balance is 99.088 parts by weight of deionized water
[0070] 2) The manufacture method of the chitosan-wrapped carrier of the B-type nano-sized arsenous acid, the steps are as follows:
[0071] (1) Preparation process of arsenous acid pre-dispersed intermediate:
[0072] 1) Take 0.12 parts by weight of Tween-80 (surfactant) and 500 parts by weight of deionized water, place them in a reaction kettle equipped with a backflow condenser, adjust the stirring speed to 2000 rpm Stir for 30 minutes under the bar;
[007...
Embodiment 3
[0085] 1) The chitosan-wrapped carrier of C-type nano-sized arsenous acid, by 100 parts by weight, consists of:
[0086] Nano-sized chitosan 0.322 parts by weight
[0087] Nano-sized arsenous acid 0.00125 parts by weight
[0088] Sodium citrate (ionotrope) 0.02 parts by weight
[0089] Castor oil polyoxyethylene ether (surfactant) 0.025 parts by weight
[0090] 0.322 parts by weight of acetic acid
[0091] Fatty alcohol polyoxyethylene ether 0.258 parts by weight
[0092] The balance is deionized water. 99.052 parts by weight
[0093] 2) The manufacture method of the chitosan-wrapped carrier of the C-type nano-sized arsenous acid, the steps are as follows:
[0094] (1) Preparation process of arsenous acid pre-dispersed intermediate:
[0095] 1), get castor oil polyoxyethylene ether (surfactant) 1.0 weight part, deionized water 1000 weight part, be placed in the reactor that is equipped with reverse flow condenser, stir speed is transferred to 2000 rev / min, in Stir for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com