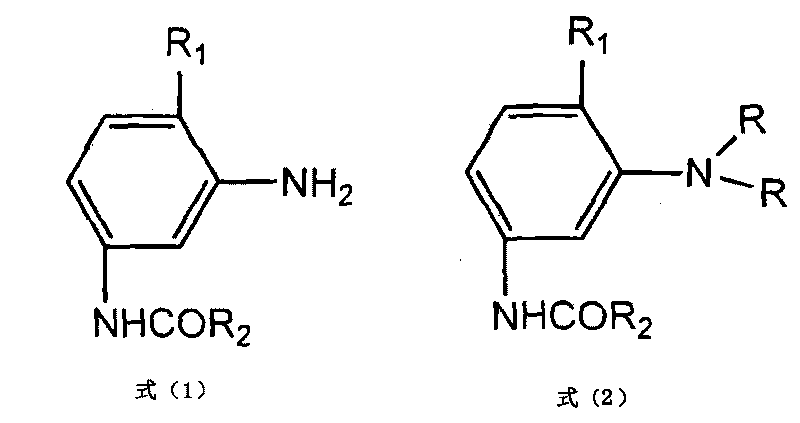
Alkylation preparation method for aniline intermediate
An intermediate and alkylation technology, applied in the field of alkylation preparation of aniline intermediates, can solve the problems of uneven product crystallization, unstable quality, low yield of alkylated products, etc. Cost-effective, the effect of preventing oil-water stratification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 108g of 3-amino-4-methoxyacetanilide (mole number 0.6) and make a slurry in 324g water, add 33g heavy magnesium oxide (mole number 0.83) as an acid-binding agent, then add 1.08g benzyl triethyl chloride Ammonium and 1.8g dispersant MF are used as catalysts, cooled to 5°C, then add 96.8g (1.5 moles) of ethyl chloride cooled to 5°C, seal, start the mixer, and heat to 90-100°C, keep warm for 18h, react After the end, cool to 80°C, and feed nitrogen into the material to drive off unreacted ethyl chloride, then add 90g of water, stir, filter, wash with water, and dry to obtain 2-methoxy-5-acetamido-N, N-diethylaniline, the yield of the obtained product is 96.6%, the purity is 99.2% (liquid chromatography), and the crystallization is uniform. The reaction formula is as follows:
[0024]
Embodiment 2
[0026] Take 110g of 3-amino-4-methoxyacetanilide (0.61 moles) and make a slurry in 255g water, add 29g of heavy magnesium oxide (0.73 moles) as an acid-binding agent, and then add 0.886g of benzyltriethyl chloride Ammonium and 1.15g dispersant (mixture of 0.8gMF and 0.35gNNO) as catalyst, cooled to 5°C, then added 92g (mole number 1.43) of ethyl chloride cooled to 5°C, sealed, started the mixer, and heated to 90~ 100°C, keep warm for 18h, after the reaction, cool to 80°C, and pass nitrogen into the material to drive off unreacted ethyl chloride, add 100g of water, stir, filter, wash with water, and dry to obtain 2-methoxy -5 Acetylamino-N, N-diethylaniline, the yield of the product obtained is 96.8%, and the purity is 99.5%.
Embodiment 3
[0028] Take 93.8g of 3-acetamidoaniline and beat in 280g of water, add 27.5g of heavy magnesium oxide as an acid-binding agent, then add 1.6g of benzyltriethylammonium chloride and 1.3g of lignin as a catalyst, cool to 5°C, Then add 101g of ethyl chloride cooled to 5°C, seal it, start the mixer, and heat to 90-100°C, keep it warm for 16h, after the reaction, cool to 80°C, and pass nitrogen into the material to drive off unreacted chlorine Add ethane, add 100 g of water, stir, filter, wash with water, and dry to obtain 3-acetamido-N,N-diethylaniline. The yield of the product is 97.2%, and the purity is 99.7%. The reaction formula is as follows:
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com