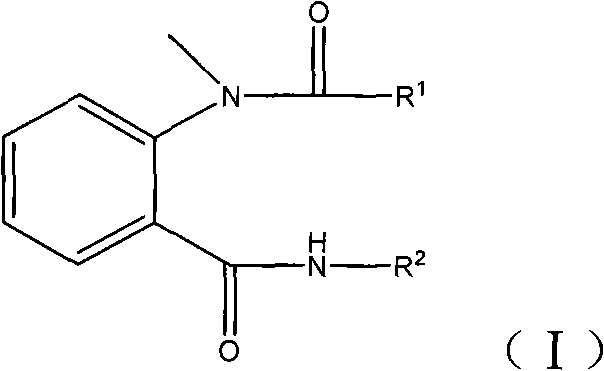
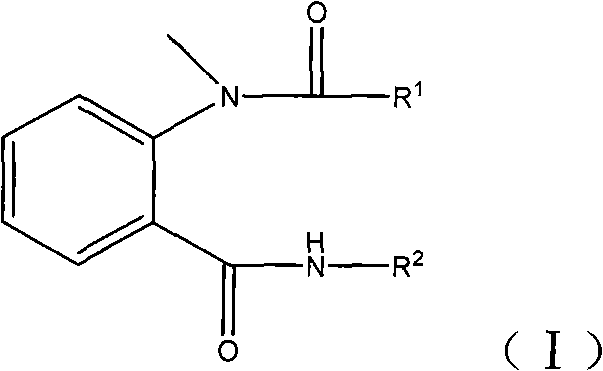
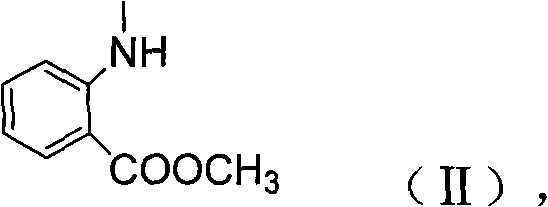
N-methyl-N-o-benzoyl aminobenzene formamide compounds as well as preparation and application thereof
A technology of o-benzamidobenzamide and benzamide, which is applied in the preparation of organic compounds, carboxylic acid amide preparation, botanical equipment and methods, etc., can solve the problems of low selectivity, restrictions on popularization and application, and direct treatment of mammals. Sexual paralysis and other problems, to achieve the effect of simple treatment process, short reaction route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of N-(2-pyridyl)-2-(N-methyl-2-methyl-4-ethylpyrazolecarboxamido)benzamide (1g)
[0022] The synthetic route of the present invention is as follows:
[0023]
[0024] where R 1 For 2-methyl-4-ethylpyrazolyl, R 2 For pyridyl.
[0025] 1) Synthesis of intermediate 2-methylamino-methyl benzoate (1d):
[0026] Add 3g (0.02mol) of methyl anthranilate and 20mL of dimethyl sulfate into a 50mL flask, react at room temperature for 12h, pour into 40mL of 1mol / L NaOH solution, and wash the water layer with ethyl acetate (30mL×3 ) extraction, combined organic layers, washed with saturated brine, anhydrous Na 2 SO 4 Drying, filtration, and precipitation under reduced pressure gave a colorless liquid, and column chromatography gave 1.69 g of colorless liquid methyl 2-methylaminobenzoate, yield: 51%;
[0027] 2) Synthesis of intermediate 2-(N-methyl-2-methyl-4-ethylpyrazole carboxamido)methyl benzoate (1e)
[0028] Add 924mg (6mmol) of 1-methyl-3-ethyl-pyrazole-5...
Embodiment 2
[0034] N-(4-trifluoromethylphenyl)-2-(N-methyl-2-methyl-4-ethylpyrazolecarboxamido)benzamide (2g):
[0035] The intermediate is synthesized with reference to the method of Example 1:
[0036] Add 5mL of anhydrous dichloromethane and 250mg (0.87mmol) 2-(N-methyl-2-methyl-4-ethylpyrazolecarboxamido)benzoic acid to a 25mL single-necked flask, and dissolve 185mg (0.9mmol) DCC and 25mg (0.2mmol) DMAP anhydrous dichloromethane solution was slowly added dropwise in a three-necked flask, after stirring at room temperature for 30min, 233mg (0.83mmol) 4-trifluoromethylaniline anhydrous dichloromethane solution was added to the flask , stirred at room temperature for 2 hours, TLC followed the reaction, and after the reaction was completed, the insoluble matter was removed by filtration under reduced pressure, and the filtrate was decomposed with 1.2mol / L NaHCO 3 After washing, desolvation under reduced pressure to obtain a light yellow solid, 132 mg of a light yellow solid was obtained ...
Embodiment 3
[0038] N-(2,6-pyrimidinyl)-2-(N-methylbenzamido)benzamide (3 g):
[0039] The intermediate is synthesized with reference to the method of Example 1:
[0040] Add 5 mL of anhydrous dichloromethane and 218 mg (0.87 mmol) of 2-(N-methyl-2-benzamido) benzoic acid in a 25 mL one-necked flask, and mix 185 mg (0.9 mmol) of DCC and 25 mg ( Slowly add 0.2mmol) DMAP anhydrous dichloromethane solution dropwise into a three-necked flask, stir at room temperature for 30min, then add 78mg (0.83mmol) 2,6-pyrimidinamine anhydrous dichloromethane solution into the flask, stir at room temperature for 2h, follow the reaction by TLC , after the reaction is finished, filter under reduced pressure to remove insoluble matter, and use 1.2mol / L NaHCO for the filtrate 3 After washing, desolvation under reduced pressure, 166 mg colloidal solid was obtained by column chromatography, yield: 37% white solid, yield: 50.1%, m.p.172~174°C, 1 H NMR (CDCl 3 , 400MHz) δ: 3.51(s, 3H), 7.09~7.77(10H), 8.66(m, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com