2-methoxyestradiol transdermal liniment and 2-methoxyestradiol transdermal patch
A technology of methoxyestradiol and transdermal patch, which is applied in the field of medicine and can solve the problems of poor water solubility, liver first-pass effect, poor curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
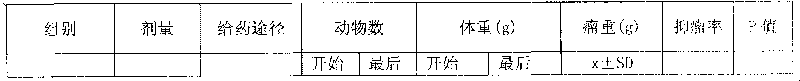
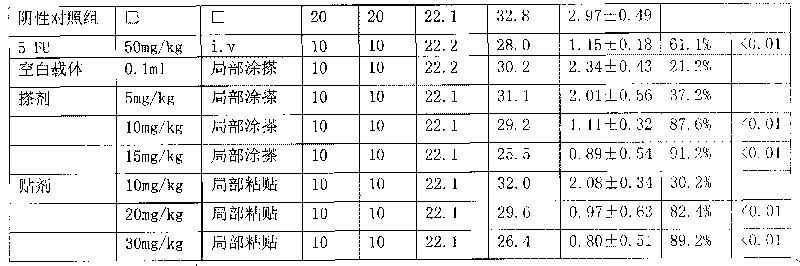
[0006] The 2-methoxyestradiol transdermal liniment of the present invention is made by mass percent: basic carrier 80%, 2-methoxyestradiol 0.8% and oil-soluble azone azone 19.2%. Add 2-methoxyestradiol and oil-soluble azone azone into the carrier, mix evenly to form the liniment of the present invention.
Embodiment 2
[0008] The 2-methoxyestradiol transdermal patch of the present invention is calculated by mass percent: basic carrier 80%, 2-methoxyestradiol 0.8%, oil-soluble azone azone 18% and hydroxypropylmethyl Made of 1.2% cellulose, add 2-methoxyestradiol and oil-soluble azone azone to the basic carrier, mix well, add hypromellose while stirring, stir well, let stand for 12 hours, make hydroxypropyl methylcellulose Methylcellulose is fully swollen to obtain a viscous solution of 2-methoxyestradiol, which is used as a drug reservoir, and the release gasket coated with polyester film is encapsulated in the rate-controlling film ethylene vinyl acetate and basement film Between the polyester films, heat seal, cut into blank patches, use a syringe to inject the viscous solution of 2-methoxyestradiol around the release circle from the unheated side of the blank patch, and then place the unheated The other side is heat-sealed to prevent liquid leakage, and finally the patch is packaged in a t...
Embodiment 3
[0010] The 2-methoxyestradiol transdermal liniment of the present invention is calculated by mass percent: basic carrier 95%, 2-methoxyestradiol 1.8% and succinic acid diisooctyl sulfonate sodium AOT 3.2% It is prepared by adding 2-methoxyestradiol and diisooctyl sodium sulfosuccinate AOT into the basic carrier, and mixing evenly to obtain the liniment of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com