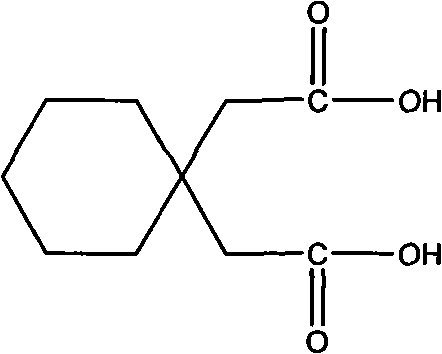
Method for utilizing dilute sulphuric acid to catalyze and hydrolyze alpha,alpha'-dicyano-1,1-cyclohexanediacetamide to prepare 1,1-cyclohexanediacetic acid
A technology of cyclohexyldiacetimide and cyclohexyldiacetic acid, which is applied in the fields of nitrile preparation and organic chemistry, can solve the problems affecting industrial application, high reaction temperature, and long reaction time, and achieve green preparation, high reaction temperature, and fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
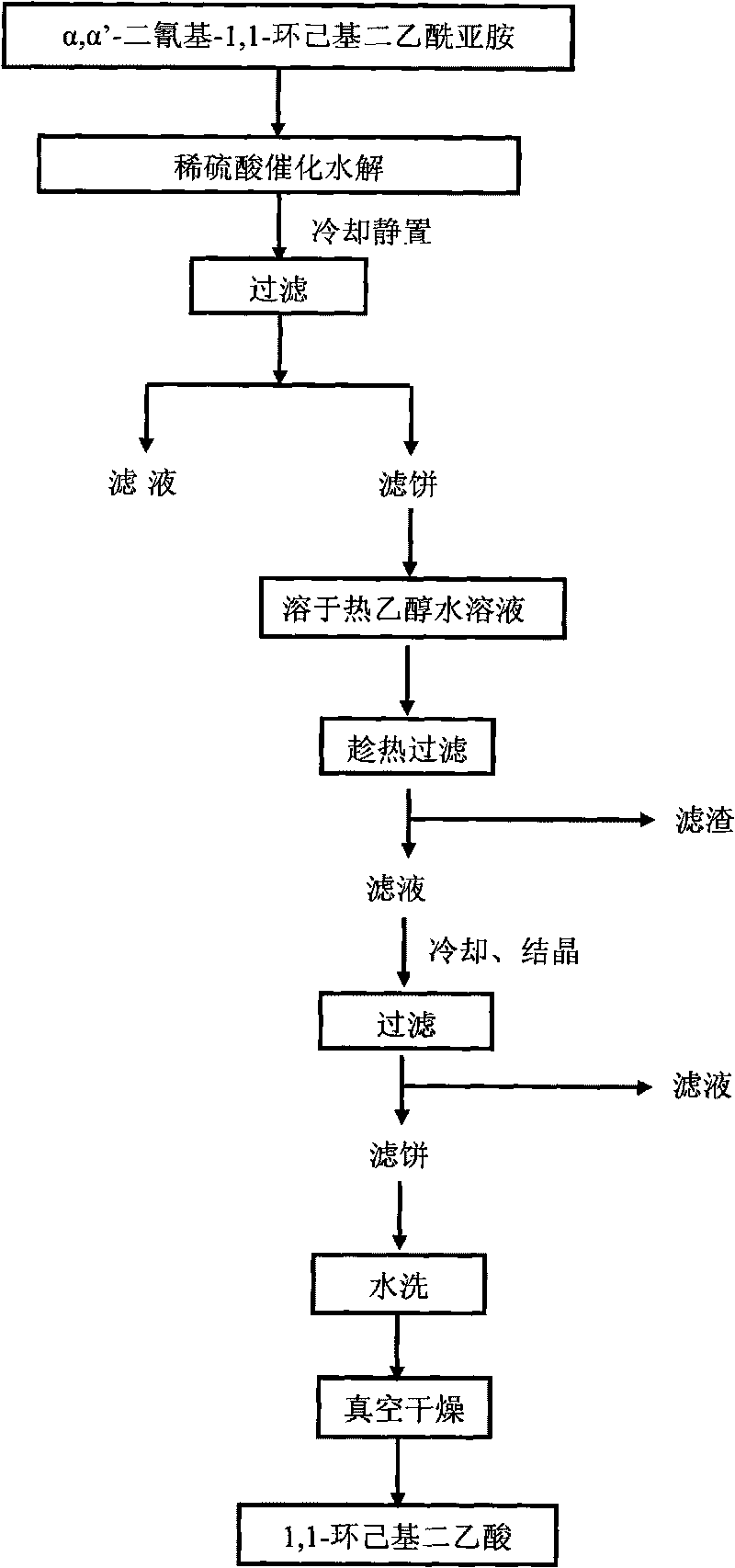
[0037] Add 360g of dilute sulfuric acid aqueous solution and 18g of α,α'-dicyano-1,1-cyclohexyl diacetylimide into a 500mL batch-type autoclave, wherein the mass concentration of sulfuric acid in the dilute sulfuric acid aqueous solution is 20%, and the dilute sulfuric acid aqueous solution The weight ratio of α, α'-dicyano-1, 1-cyclohexyldiacetimide is 20. Turn on stirring, heat up to boiling under normal pressure, open the exhaust valve for 2 minutes, and use water vapor to remove the air in the kettle; close the exhaust valve, continue to heat up to 250°C for 10 minutes for hydrolysis; the reaction solution is cooled and left to stand, then filtered to obtain a filter cake Dissolve the filter cake in an aqueous solution containing 20% ethanol (volume concentration) at 50°C, and filter while it is hot; the filtrate crystallizes after cooling, and obtains the product 1,1-cyclohexyldiacetic acid 10.3g after filtering, washing with water, and vacuum drying, the product The pu...
Embodiment 2
[0039] Add 330g of dilute sulfuric acid aqueous solution and 22g of α,α'-dicyano-1,1-cyclohexyl diacetylimide in a 500mL batch-type autoclave, wherein the mass concentration of sulfuric acid in the dilute sulfuric acid aqueous solution is 15%, dilute sulfuric acid The weight ratio of aqueous solution to α,α'-dicyano-1,1-cyclohexyl diacetimide is 15. Turn on stirring, heat up to boiling under normal pressure, open the exhaust valve for 4 minutes, and use water vapor to remove the air in the kettle; close the exhaust valve, continue to heat up to 230°C for 25 minutes for hydrolysis; the reaction solution is cooled and left to stand, then filtered to obtain a filter cake Dissolve the filter cake in an aqueous solution containing 30% ethanol (volume concentration) at 55°C, and filter while it is hot; the filtrate crystallizes after cooling, and obtains the product 1,1-cyclohexyldiacetic acid 15.1g after filtering, washing with water, and vacuum drying, the product The purity by HP...
Embodiment 3
[0041]Add 300g of dilute sulfuric acid aqueous solution and 30g of α,α'-dicyano-1,1-cyclohexyl diacetylimide in a 500mL batch-type autoclave, wherein the mass concentration of sulfuric acid in the dilute sulfuric acid aqueous solution is 10%, dilute sulfuric acid The weight ratio of aqueous solution to α,α'-dicyano-1,1-cyclohexyl diacetimide is 10. Turn on stirring, heat up to boiling under normal pressure, open the exhaust valve for 5 minutes, and use water vapor to remove the air in the kettle; close the exhaust valve, continue to heat up to 210°C for 45 minutes for hydrolysis; the reaction solution is cooled and left to stand, then filtered to obtain a filter cake Dissolve the filter cake in an aqueous solution containing 40% ethanol (volume concentration) at 60°C, and filter while it is hot; the filtrate crystallizes after cooling, and obtains the product 1,1-cyclohexyldiacetic acid 22.6g after filtering, washing with water, and vacuum drying. The purity by HPLC analysis w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com