Method for preparing cilazapril intermediate
A technology of intermediates and compounds, which is applied in the field of cilazapril synthesis technology, can solve the problems of reducing the yield of the final product cilazapril, difficulty in separation and purification, and instability, so as to achieve economical and practical production methods and avoid separation and purification , the effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
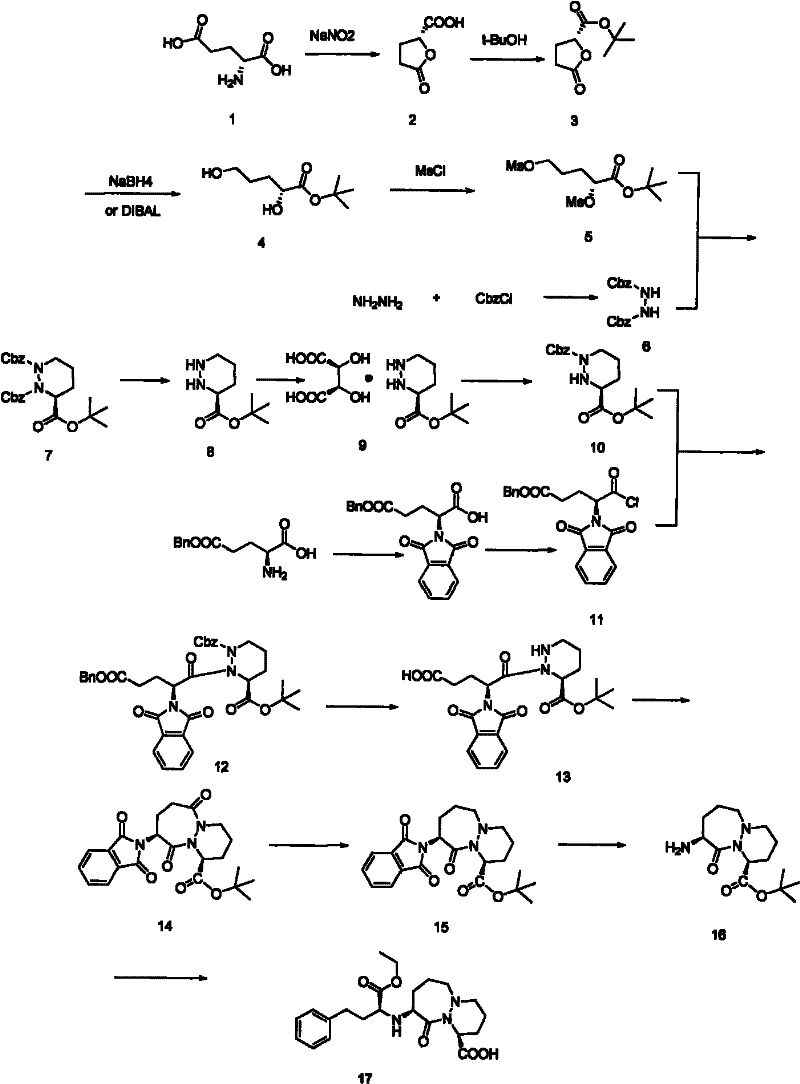
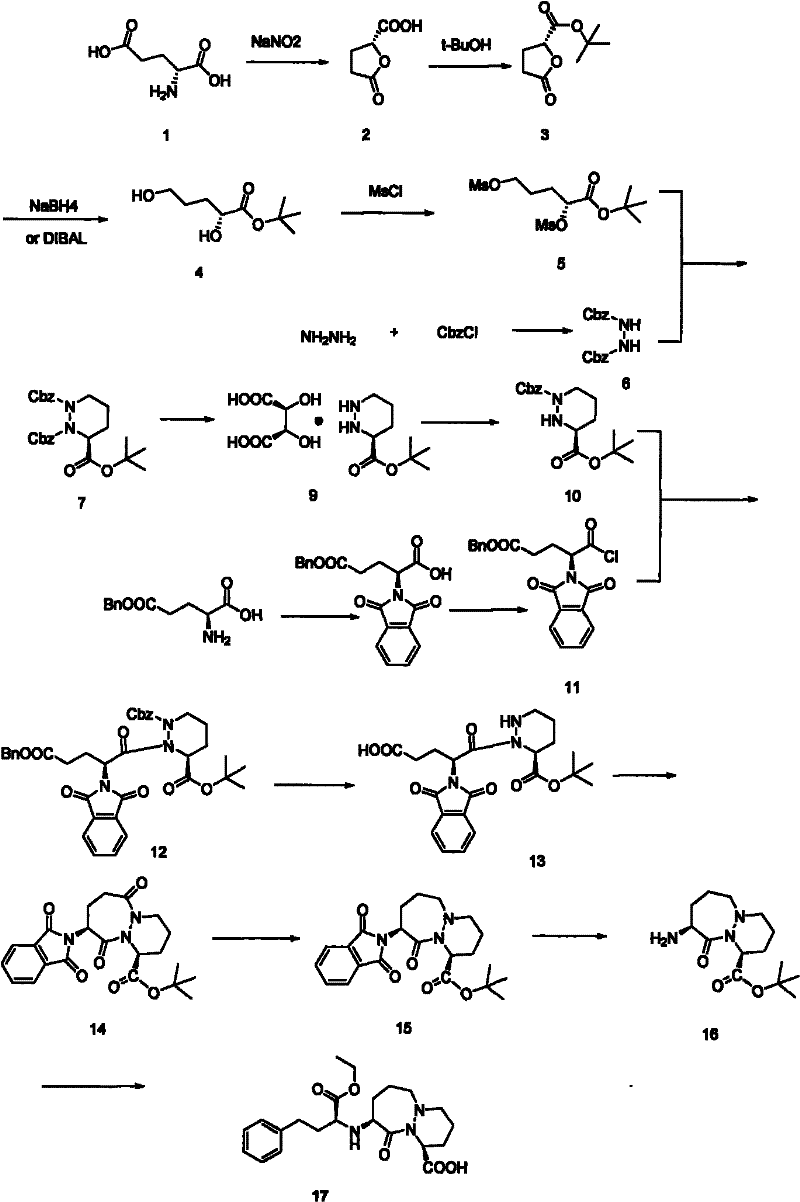
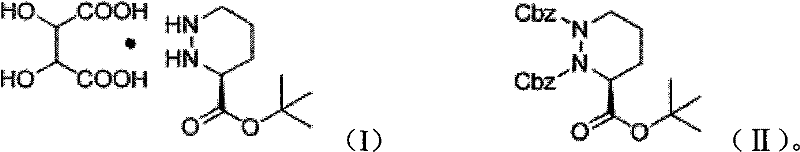
[0021] Add (1S)-1,2-dibenzyloxycarbonyl-3-tert-butylhexahydropyridazine-1,2,3-tricarboxylate 90g (0.198mol), L-tartaric acid 35.6 (0.237mol), tetrahydrofuran (THF) solvent 0.75L, Pd / C (5% Pd) 9.0g, control reaction temperature is 70 ℃, hydrogen pressure is 4atm, react until the hydrogen pressure no longer drops, the reaction material is cooled to 0°C, filter, wash the filter cake with ethyl acetate, and then wash the filter cake three times with a mixture of methanol and water with a volume ratio of 3:1, collect the filtrate, and rotary evaporate to obtain a white solid. The obtained white solid was dissolved in n-butanol, and crystallized slowly to obtain 54 g of cilazapril intermediate (1S)-hexahydropyridazine-3-tert-butylcarboxylate L-tartrate in (S) configuration. The calculated yield of the cilazapril intermediate was 81%.
Embodiment 2
[0023] Add (1S)-1,2-dibenzyloxycarbonyl-3-tert-butylhexahydropyridazine-1,2,3-tricarboxylate 90g (0.198mol), L-tartaric acid 35.6 g (0.237mol), tetrahydrofuran (THF) solvent 0.75L, Pd / C (5%Pd) 9.0g, the control reaction temperature is 65 ℃, and the hydrogen pressure is 5atm, reacts until the hydrogen pressure no longer drops, and the reaction mass is cooled to 0°C, filtered, washed the filter cake with ethyl acetate, and washed the filter cake three times with a mixture of methanol and water at a volume ratio of 3:1, collected the filtrate, and rotovapped to obtain a white solid. The resulting white solid was dissolved in n-butanol and crystallized slowly to obtain the (S) configuration of Cilazapril intermediate (1S)-hexahydropyridazine-3-tert-butylcarboxylate L-tartrate (51g) . The calculated yield of cilazapril intermediate was 76.7%.
Embodiment 3
[0025] Add (1S)-1,2-dibenzyloxycarbonyl-3-tert-butylhexahydropyridazine-1,2,3-tricarboxylate 120g (0.264mol), L-tartaric acid 39.6 g (0.264mol), tetrahydrofuran (THF) solvent 1L, Pd / C (5% Pd) 12g, control the reaction temperature to 70°C, the hydrogen pressure to 4atm, react until the hydrogen pressure no longer drops, and cool the reaction materials to 0°C , filtered, washed the filter cake with ethyl acetate, and washed the filter cake three times with a mixture of methanol and water with a volume ratio of 3:1, collected the filtrate, and rotovapped to obtain a white solid. The resulting white solid was dissolved in n-butanol and slowly crystallized to obtain the (S) configuration of Cilazapril intermediate (1S)-hexahydropyridazine-3-tert-butylcarboxylate L-tartrate (70g) . The calculated yield of cilazapril intermediate was 78.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com