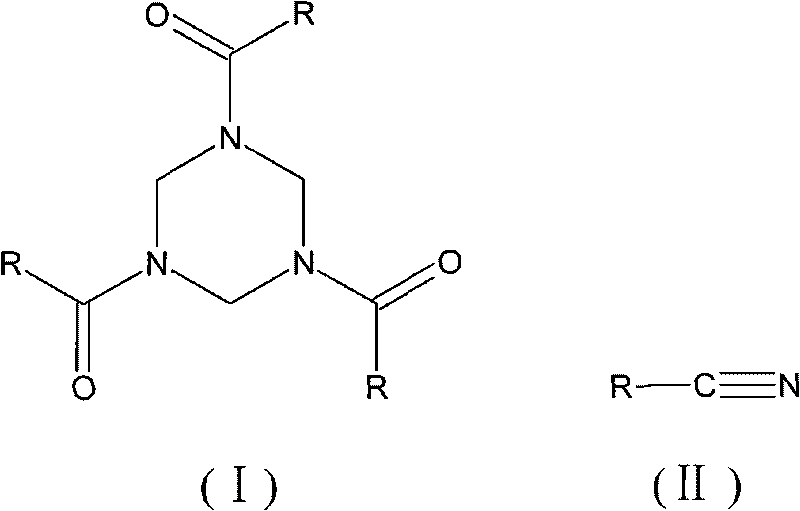
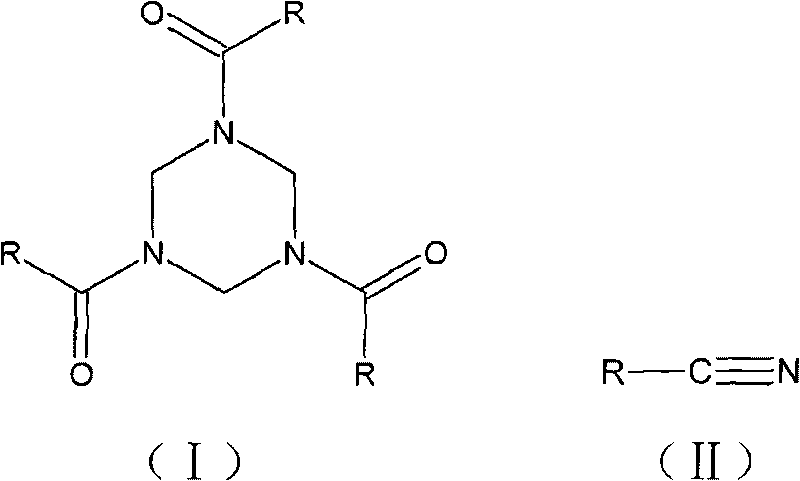
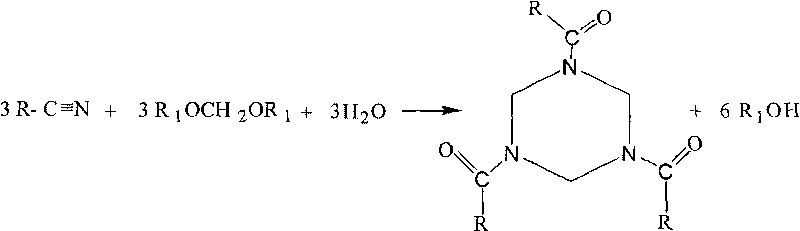Synthesis method of 1,3,5-tri-substituted perhydro-s-triazine
A synthesis method and technology of s-triazine, applied in 1 field, can solve problems such as low yield of hexahydro-s-triazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] a. Preparation of carrier
[0020] After mixing 0.20 mol of phenyltrimethoxysilane and 0.80 mol of tetraethoxysilane, 280 mL of ethanol was added, and vigorously stirred at 60° C. for 4 h. Then add 135mL cyclohexane, 280mL water and 0.01mol dodecylamine to the above mixed solution, mix well, place it at 80°C to age for 3 days, the reaction solution appears white precipitate, filter, filter out the solid, in vacuum Dry at 80°C for 10 hours under the same conditions, and bake at 350°C for 4 hours to obtain a phenyl-bonded polysiloxane material.
[0021] Introduction of b sulfonic acid group
[0022] After soaking the phenyl-bonded polysiloxane material in tetrachloroethane solution overnight, add 15% sulfur trioxide solution in tetrachloroethane, stir at 120° C. for 5 h, and then cool naturally to room temperature. Filter, wash until the pH is 6-7, and dry at 120° C. for 8 hours to obtain a catalyst phenylsulfonic acid functionalized silicon-based material.
Embodiment 1
[0024] Synthesis of 1,3,5-triphenylacetylhexahydro-s-triazine
[0025] In a three-neck flask equipped with mechanical stirring, a thermometer and a reflux condenser, add 0.3 mol of phenylacetonitrile, 0.075 mol of methanol formal, 70 mL of organic solvent 1,2-dichloroethane, and 0.9 g of catalyst, and heat to reflux under stirring. Continue to stir the reaction for 6h, stop heating, and the reaction ends. The reaction solution was filtered while it was hot, and after the solid catalyst was separated, the filtrate was distilled off under reduced pressure to remove the organic solvent 1,2-dichloroethane, cooled to room temperature, allowed to stand for 11 hours, and filtered to obtain 1,3,5-triphenylacetylhexa Hydrogen-s-triazine, the yield is 98.4%.
[0026] Structure Identification
[0027] Molecular formula: C 27 h 27 N 3 o 3
[0028] Elemental analysis: theoretical value: C 73.45 H 6.160 N 9.52;
[0029] Found: C 73.49 H 6.153 N 9.48.
[0030] Proton NMR spectrum: δ...
Embodiment 2
[0035] Synthesis of 1,3,5-triacetylhexahydro-s-triazine
[0036] In a three-neck flask equipped with a mechanical stirrer, a thermometer and a reflux condenser, add 0.5 mol of acetonitrile, 0.1 mol of ethanol formal, 80 mL of organic solvent 1,2-dichloroethane, and 0.3 g of catalyst, and heat to reflux while stirring, and continue Stir for 10h, stop heating, and the reaction ends. The reaction solution was filtered while it was hot, and after the solid catalyst was separated, the filtrate was decompressed to remove the solvent, cooled to room temperature, and allowed to stand for 10 hours, and the solid was precipitated by filtration to obtain the target product 1,3,5-triacetylhexahydro-s-triazine, yield was 96.3%.
[0037] Structure Identification
[0038] Molecular formula: C 9 h 15 N 3 o 3
[0039] Elemental analysis: theoretical value: C 50.70 H 7.042 N 19.72;
[0040] Found: C 50.61 H 7.031 N 19.61.
[0041] Proton NMR spectrum: δ: 2.15 (s, 9H) 5.23 (s, 6H).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com