Method for preparing freeze-drying particle preparation for injection
A technology for injection and water for injection, which is applied in the field of medicine, can solve the problems of low drug loading, poor stability, and large amount of auxiliary materials in microparticle preparations, and achieve the effects of reduced preparation cost, less application of auxiliary materials, and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 paclitaxel injection freeze-dried microparticle preparation
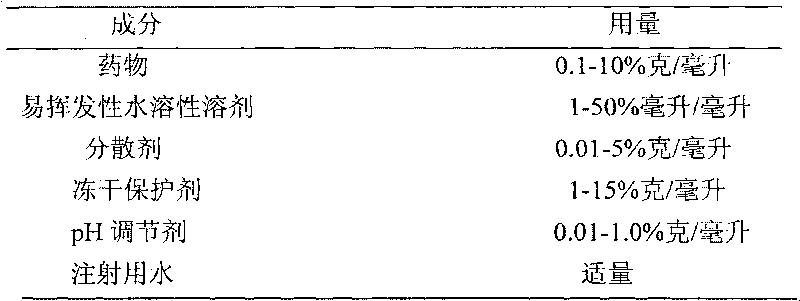
[0031] Weigh 1.0 g of paclitaxel into 5 ml of absolute ethanol, heat and stir at 60°C to dissolve, and obtain a molecular drug solution, disperse it in 100 ml of water for injection at 40°C, and incubate at 40°C for 10 minutes to obtain the drug Soft aggregate suspension; add 0.1 g of egg yolk lecithin, 2.0 g of sucrose, 1.0 g of human serum albumin, and 0.4 g of citric acid, shear to disperse, apply mechanical energy to open the aggregate with high-pressure homogenization, and the homogenization pressure is 12000 psi , 45°C rotary evaporation to remove absolute ethanol, filter through a 0.22 μm microporous membrane, aliquot, lyophilize, and seal to obtain paclitaxel injection lyophilized microparticle preparation.
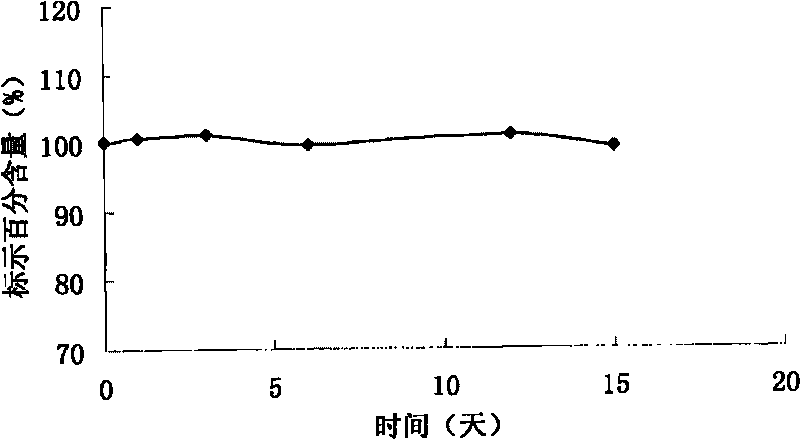
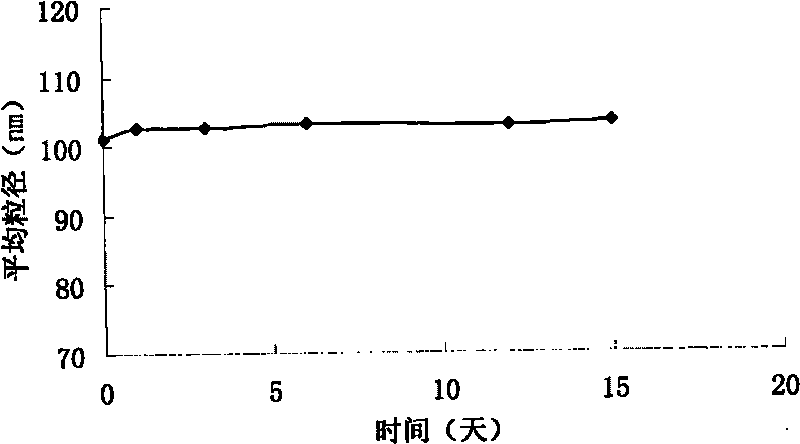
[0032] The average particle size of the lyophilized preparation after reconstitution was 101 nm.
Embodiment 2
[0033] Embodiment 2 paclitaxel injection freeze-dried microparticle preparation
[0034] Weigh 2.0 grams of paclitaxel into 10 ml of absolute ethanol, heat and stir at 70°C to dissolve, and obtain a molecular drug solution; inject the molecular drug solution into 60 ml of water for injection at 40°C, and incubate at 40°C for 60 minutes , to obtain drug soft aggregate suspension; weigh 0.1 g of egg yolk lecithin, 3.0 g of mannitol, 2.0 g of human serum albumin, and 0.6 g of citric acid, put them in 40 ml of water for injection, shear at 40°C to disperse, and score Powder dispersion; mix the dispersant dispersion with drug soft aggregate suspension, apply mechanical energy to open the aggregate with high-pressure homogenization, the homogenization pressure is 15000psi, 55°C rotary evaporation removes absolute ethanol, 0.22μm microporous membrane Filtrating, subpackaging, freeze-drying, and sealing to obtain the freeze-dried microparticle preparation of paclitaxel for injection. ...
Embodiment 3
[0036] Embodiment 3 paclitaxel injection freeze-dried microparticle preparation
[0037] Weigh 0.5 g of paclitaxel into 3 ml of absolute ethanol, heat and stir at 50°C to dissolve, and obtain a molecular drug solution; inject the molecular drug solution into 50 ml of water for injection at 80°C, and incubate at 80°C for 30 minutes , to obtain drug soft aggregate suspension; weigh 0.8 grams of human serum albumin, 5.0 grams of mannitol, and 0.4 grams of lactic acid, put them in 50 milliliters of water for injection, stir at 60 ° C to dissolve, and obtain a dispersant dispersion; Mix with drug soft aggregate suspension, use high-pressure homogenization to apply mechanical energy to open the aggregate, the homogenization pressure is 8000psi, 60°C rotary evaporation removes absolute ethanol, 0.22μm microporous membrane filtration, sub-packaging, freeze-drying, sealing , to obtain the freeze-dried microparticle preparation of paclitaxel for injection.
[0038] The average particle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com