Method for preparing living paratyphoid vaccine for piglets and product thereof
A technology for piglet paratyphoid and live vaccine, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, antibacterial drugs, etc., and can solve the problems of low yield, difficult production, and low bacterial count
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
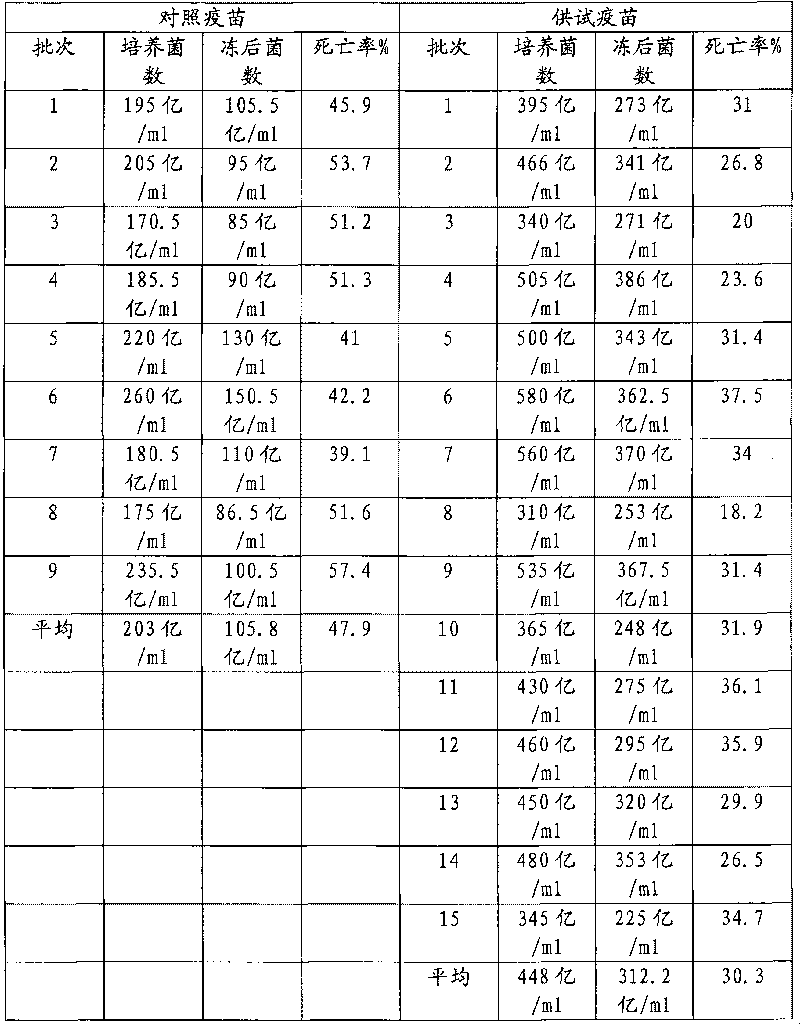
Examples
Embodiment 1
[0028] 1. Preparation of primary strains:
[0029] Dilute the strain of Salmonella choleraesuis (purchased from the China Veterinary Drug Control Institute) with ordinary broth or culture and propagate it in ordinary broth, inoculate on ordinary agar plate, culture at 37°C for 18-20h, and select colonies that are round, with neat edges and protruding , 5-10 translucent, slightly moist smooth medium-sized colonies, mixed and inoculated on several branches of ordinary agar slant, cultured at 37°C for 24 hours, tested purely, and used 1 / 500 acridine yellow solution to do the slide agglutination test to check that it was qualified. As a first-class strain, it should be stored at 2-8°C, and it should not exceed 2 months. It can be transplanted 1-2 times during this period, and it can be subcultured on the culture medium for no more than 5 generations.
[0030] 2. Preparation of secondary strains:
[0031] Hook the first-grade strains and inoculate them into ordinary broth agar fla...
Embodiment 2
[0035] 1. Preparation of primary strains:
[0036]Dilute the strains of Salmonella choleraesuis (please give the commercial purchase channel of the strains of Salmonella choleraesuis) with ordinary broth or after culture and propagation in ordinary broth, inoculate the common agar plate, culture at 37°C for 18-20h, select the colony as a circle 5-10 medium-sized colonies with neat shape, neat edges, protruding, translucent, and slightly moist smooth, mixed and inoculated on several branches of ordinary agar slant, cultured at 37°C for 24 hours, tested purely, and made glass with 1 / 500 acridine yellow solution The sheet agglutination test is qualified, as a first-class strain, it should be stored at 2-8°C, and it should not exceed 2 months. It can be transplanted 1-2 times during this time, and it can be subcultured on the medium for no more than 5 generations.
[0037] 2. Preparation of secondary strains:
[0038] Hook the first-grade strains and inoculate them in common brot...
Embodiment 3
[0042] 1. Preparation of primary strains:
[0043] Dilute the strains of Salmonella choleraesuis (please give the commercial purchase channel of the strains of Salmonella choleraesuis) with ordinary broth or after culture and propagation in ordinary broth, inoculate the common agar plate, culture at 37°C for 18-20h, select the colony as a circle 5-10 medium-sized colonies with neat shape, neat edges, protruding, translucent, and slightly moist smooth, mixed and inoculated on several branches of ordinary agar slant, cultured at 37°C for 24 hours, tested purely, and made glass with 1 / 500 acridine yellow solution The sheet agglutination test is qualified, as a first-class strain, it should be stored at 2-8°C, and it should not exceed 2 months. It can be transplanted 1-2 times during this time, and it can be subcultured on the medium for no more than 5 generations.
[0044] 2. Preparation of secondary strains:
[0045] Hook the first-grade strains and inoculate them into ordinary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com