Carbonyl sulfur hydrolyst prepared by using binary type hydrotalcite as precursor and method for preparing same
A hydrolysis catalyst, hydrotalcite technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., to achieve low operating temperature, high desulfurization accuracy, and energy saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
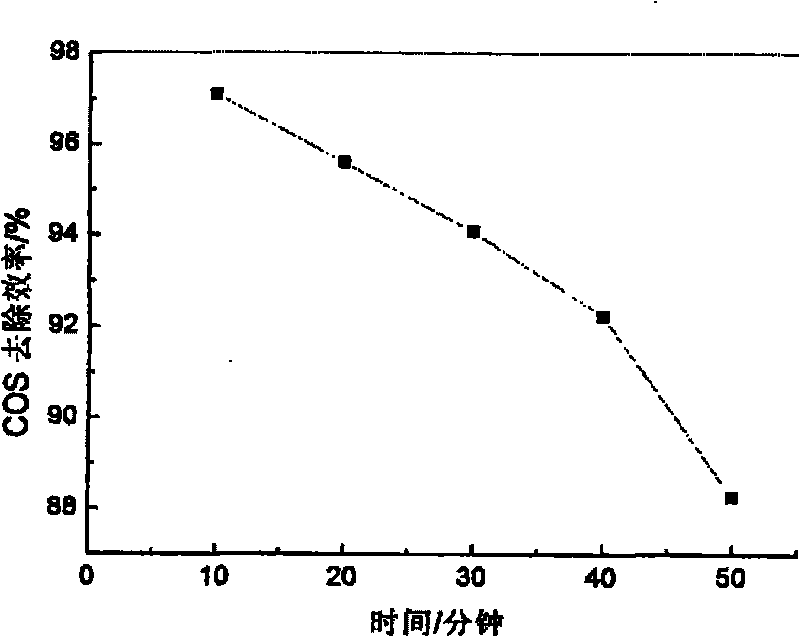
Embodiment 1
[0026] (1) Weigh 12.8205gMg(NO 3 ) 2 ·6H 2 O and 9.3782gAl(NO 3 ) 3 9H 2 O was dissolved in distilled water to make solution A, so that the total metal mole was 0.075mol, and n(Mg 2+ ):n(Al 3+ )=2. Take NaOH, Na 2 CO 3 As a precipitating agent, take 5.2995gNa 2 CO 3 (0.05mol) and 7g NaOH were dissolved in distilled water to form solution B.
[0027] (2) Move the prepared A solution to a separatory funnel and drop the A solution into the B solution at a rate of 3.6 mL / min under room temperature and mechanical stirring, and control the pH of the solution at the end of the addition to be 10. After the dropwise addition, the stirring was continued for 30 min, and finally a suspension was obtained.
[0028] (3) Crystallize the suspension obtained in step (2) in a water bath at 50° C. for 12 hours.
[0029] (4) The product obtained by crystallization is suction-filtered, and washed with distilled water to make it neutral. The obtained product was put into an oven and d...
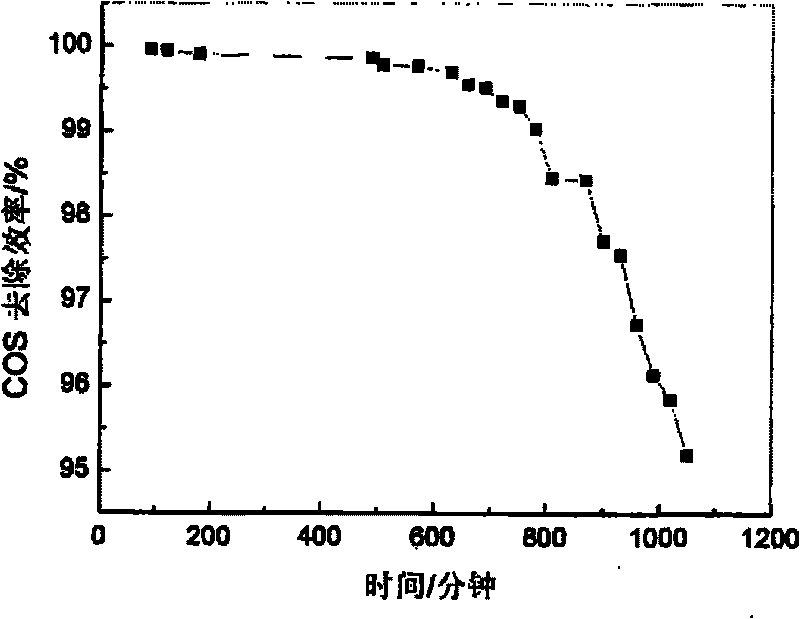
Embodiment 2
[0032] (1) Weigh 16.3575gNi(NO 3 ) 2 ·6H 2 O and 7.575gFe(NO 3 ) 3 9H 2 O was dissolved in distilled water to make solution A, so that the total metal mole was 0.075mol, and n(Ni 2+ ):n(Fe 3+ )=3. Take NaOH, Na 2 CO 3 As a precipitating agent, take 5.2995gNa 2 CO 3 (0.05mol) and 6.75g NaOH were dissolved in distilled water to form solution B.
[0033] (2) Move the prepared A solution to a separatory funnel and drop the A solution into the B solution at a rate of 3.6 mL / min under room temperature and mechanical stirring, and control the pH of the solution at the end of the addition to be 9. After the dropwise addition, the stirring was continued for 30 min, and finally a suspension was obtained.
[0034] (3) Crystallize the suspension obtained in step (2) in a water bath at 50° C. for 12 hours.
[0035] (4) The product obtained by crystallization is suction-filtered, and washed with distilled water to make it neutral. The obtained product was put into an oven and ...
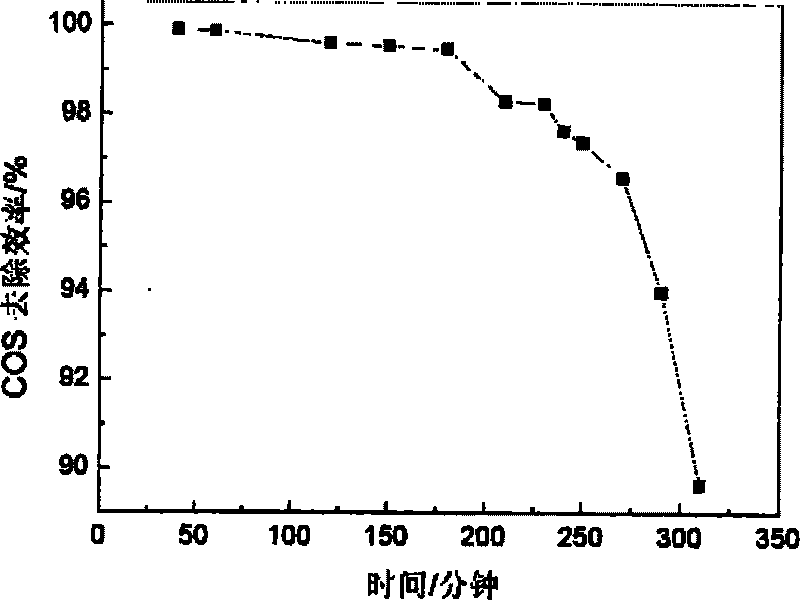
Embodiment 3
[0038] (1) Weigh 14.4231gMg(NO 3 ) 2 ·6H 2 O and 7.575g Fe(NO 3 ) 3 9H 2 O was dissolved in distilled water to make solution A, so that the total metal mole was 0.075mol, and n(Mg 2+ ):n(Fe 3+ )=3. Take NaOH, Na 2 CO 3 As a precipitating agent, take 5.2995gNa 2 CO 3 (0.05mol) and 6.75g NaOH were dissolved in distilled water to form solution B.
[0039] (2) Transfer the prepared A solution to a separatory funnel for titration. Under the conditions of room temperature and mechanical stirring, drop solution A into solution B at a rate of 3.6 mL / min, and control the pH of the solution at the end of the addition to be 9. After the dropwise addition, the stirring was continued for 30 min, and finally a suspension was obtained.
[0040] (3) Crystallize the suspension obtained in step (2) in a water bath at 50° C. for 12 hours.
[0041] (4) The product obtained by crystallization is suction-filtered, and washed with distilled water to make it neutral. The obtained produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com