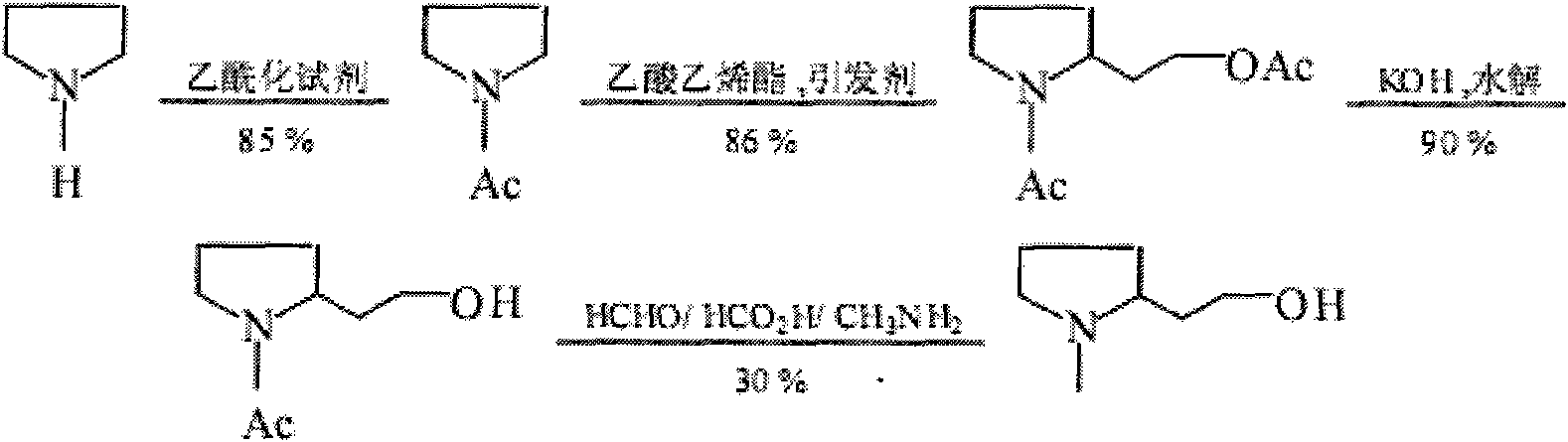
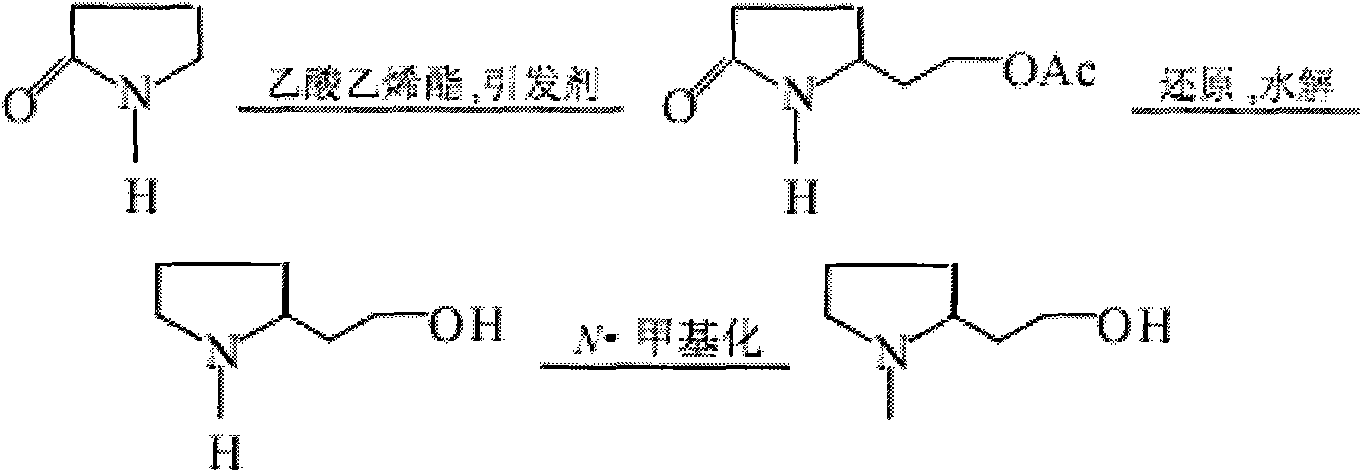
Synthesis method of N-methyl-2-hydroxyethyl hydroxyethyl
A technology of hydroxyethylpyrrolidine and methylpyrrolidone is applied in the synthesis field of N-methyl-2-hydroxyethylpyrrolidine, can solve the problems of high production cost, low yield and the like, and achieves short synthesis route and reaction Mild conditions and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take a 1000ml three-neck flask, add 140ml of dimethyl sulfate and 140ml of N-methylpyrrolidone, raise the temperature to 60°C, and keep the temperature at 60-75°C for 3 hours. After completion, cool to 5°C, add 145ml of ethyl cyanoacetate dropwise, and react below 20°C for 3 hours, filter, and dry to obtain about 240g of intermediate (I), with a yield of 86.7%.
Embodiment 2
[0031] Take a 1000ml three-neck flask, add 140ml dimethyl sulfate and 140ml N-methylpyrrolidone, raise the temperature to 50°C, and keep it at 50-60°C for 3 hours. After completion, cool to 5°C, add 145ml of ethyl cyanoacetate dropwise, and react below 20°C for 3 hours, filter, and dry to obtain about 205g of intermediate (I), with a yield of 74.0%.
Embodiment 3
[0033] Take a 1000ml three-neck flask, add 140ml of dimethyl sulfate and 140ml of N-methylpyrrolidone, raise the temperature to 75°C, and keep it at 75-80°C for 3 hours. Finished, then cooled to 5°C, added dropwise 145ml of ethyl cyanoacetate, after dropping, reacted below 20°C for 3 hours, filtered, and dried to obtain about 130g of intermediate (I), with a yield of 46.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com