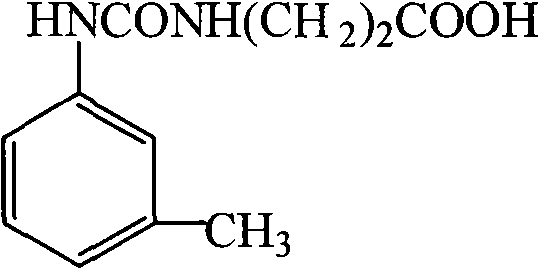
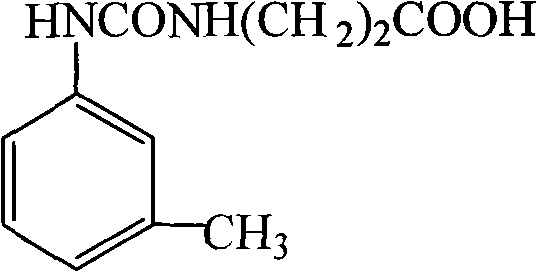
Specific antibody against pesticide meta-tolyl-N-methylcarbamate
A kind of Methiocarb, specific technology, applied in the direction of specific peptides, from serum immunoglobulins, decapeptides, etc., can solve the problems of long detection time, expensive instruments, complicated pretreatment, etc., and achieve good specificity and sensitivity, similar to high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Meprocarb artificial antigen and antibody preparation
[0042] 1 Pesticide Methiocarb Hapten Synthesis
[0043] (1) Synthesis of hapten 1-(3-carboxyethyl)-3-m-tolylurea (SN)
[0044]
[0045] Weigh 445mg of β-alanine and 665mg of 1-m-toluene isocyanate into a 100mL round bottom flask, add 50mL of anhydrous tetrahydrofuran solution, stir at room temperature overnight, filter, and pass the obtained filtrate through a silica gel column, using a ratio of 1:40 Methanol: dichloromethane was eluted for 1 hour, then the eluent was replaced with pure methanol, the column was washed for 0.5 hours, all the eluate was collected, and vacuum-dried to obtain the Methiocarb hapten 1-(3-carboxyethyl) -3-m-tolylurea.
[0046] (2) Synthesis of 1-(3-carboxyethyl)-3-m-tolylurea activated ester
[0047]
[0048] Take 110mg of the hapten 1-(3-carboxyethyl)-3-m-tolylurea synthesized by the above method, and place 71mg of N-hydroxysuccinimide in a 25ml round bottom flask a...
Embodiment 2
[0060] Embodiment 2 Meprocarb artificial antigen and antibody preparation
[0061] 1 Technical route for the synthesis of the pesticide Methiocarb hapten:
[0062] (1) Synthesis of hapten 1-(3-carboxyethyl)-3-m-tolylurea (SN)
[0063]
[0064] Weigh 460mg of β-alanine and 675mg of 1-m-toluene isocyanate into a 100mL round bottom flask, add 50mL of anhydrous tetrahydrofuran solution, stir at room temperature overnight, filter, and the resulting filtrate is purified by silica gel column chromatography with a ratio of 1: 40 methanol:dichloromethane was eluted for 1 hour, then the eluent was replaced with pure methanol, the column was washed for 0.5 hours, all the eluate was collected, and vacuum-dried to obtain the amethancarb hapten 1-(3-carboxyethyl base)-3-m-tolylurea.
[0065] (2) Synthesis of 1-(3-carboxyethyl)-3-m-tolylurea activated ester
[0066]
[0067] Take 120mg of the hapten 1-(3-carboxyethyl)-3-m-tolylurea synthesized by the above method, and place 73mg of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com