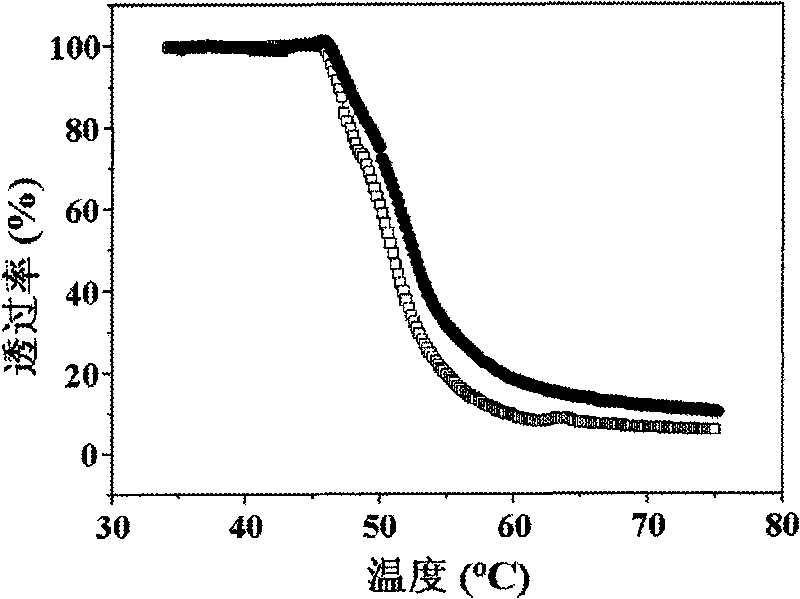
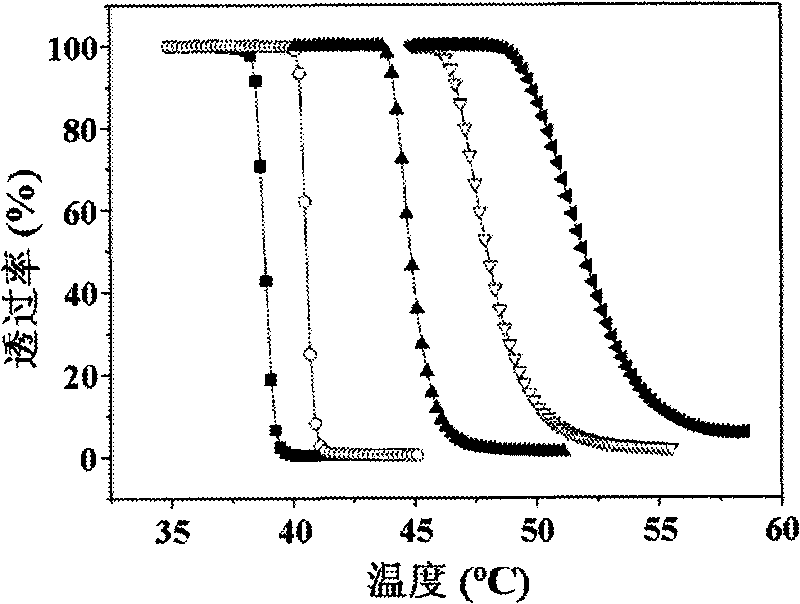
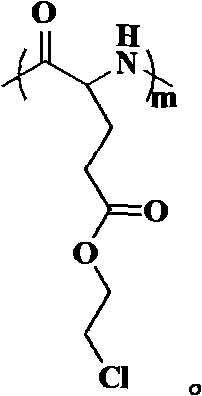
Chloroethyl alcohol functional poly (L-glutamic acid) homopolymer and random copolymer and preparation method and application thereof
A technology of random copolymer and glutamic acid, which is applied in the field of chlorohydrin functionalized poly(L-glutamic acid) homopolymerization and random copolymer and its preparation method and application field, can solve the problem of non-biodegradable and difficult preparation process Control, limit and other issues, to achieve the effect of non-toxic side effects, good biocompatibility and degradability, and improved intelligence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of different molecular weight poly(γ-2-chloroethyl-L-glutamate)
[0032] Weigh 3 parts of 2.356g (0.01mol) of γ-2-chloroethyl-L-glutamate-N-internal carboxylic acid anhydride monomer respectively, add them to 3 dry reaction flasks, add 5mL of anhydrous N,N-Dimethylformamide dissolves the monomer. Add 0.00033 mol, 0.00020 mol and 0.00014 mol of n-hexylamine under stirring, and then continue to react the solution at 25°C for 72 hours. Dry under vacuum for 24 hours to obtain three kinds of poly(γ-2-chloroethyl-L-glutamate) with different molecular weights. The obtained products are shown in Table 1.
[0033] Table 1 Preparation of poly(γ-2-chloroethyl-L-glutamate) with different molecular weights
[0034] experiment number
[0035] In the above table, A / I is the molar ratio of γ-2-chloroethyl-L-glutamate-N-internal carboxylic anhydride to n-hexylamine; Mn is poly(γ-2-chloroethyl-L- Glutamate) number average molecular weight, by 1 H ...
Embodiment 2
[0036] Embodiment 2: the preparation of poly(gamma-2-chloroethyl-L-glutamate)
[0037] Weigh 2.356g (0.01mol) of γ-2-chloroethyl-L-glutamate-N-internal carboxylic acid anhydride monomer into a dry reaction flask, add 5mL of anhydrous N,N-dimethylformaldehyde Amides dissolve the monomers. Add 0.00010 mol of hexamethylenediamine under stirring, and then continue to react the solution at 25°C for 72h. After the reaction, the reaction system is settled with 50mL ether, filtered, washed with ether three times, and then vacuum-dried at 25°C for 24h. Poly(γ-2-chloroethyl-L-glutamate) was obtained with a reaction yield of 75.3%.
Embodiment 3
[0038] Embodiment 3: the preparation of poly(gamma-2-chloroethyl-L-glutamate)
[0039] Weigh 2.356g (0.01mol) of γ-2-chloroethyl-L-glutamate-N-internal carboxylic acid anhydride monomer into a dry reaction flask, add 50mL of anhydrous 1,4-dioxane Dissolve the monomer. Add 0.00010 mol of n-hexylamine under stirring, and then continue to react the solution at 25°C for 72h. After the reaction, the reaction system is settled with 500mL ether, filtered, washed with ether three times, and vacuum-dried at 25°C for 24h to obtain Poly(γ-2-chloroethyl-L-glutamate), the reaction yield was 76.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com