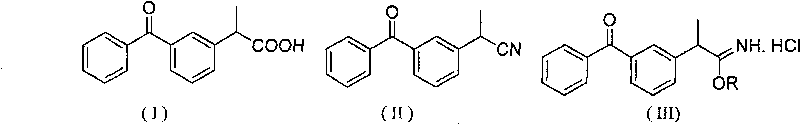
Synthesis method of ketoprofen
A synthesis method and technology of nitrile ethyl benzophenone, applied in the field of ketoprofen synthesis, can solve the problems of high hydrolysis cost of 3-nitrile ethyl benzophenone, difficult post-processing, long reaction time and the like, and achieve The effect of short reaction time, long time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
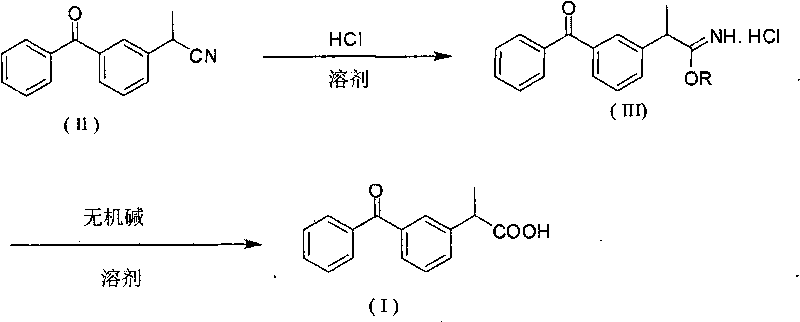
[0020] Dissolve 23.5g (0.1mol) of 3-nitrile ethyl benzophenone in 200ml of methanol, pour it into a three-necked flask, feed HCl gas, and react at room temperature for 1.5 hours. TLC will track the reaction. When the raw material point disappears, the reaction ends 8g (0.2mol) NaOH and 100ml water were added to the solution, and the mixture was refluxed at 70°C for 2 hours. The reaction liquid was cooled to room temperature, and the pH value was adjusted to 3.5 with 1 mol / L hydrochloric acid, and a solid was precipitated. Filtrate through filter paper, put the filter cake into an oven to dry, recrystallize the crude product with methanol (300 mL), filter through filter paper, put the filter cake into an oven to dry, and obtain 22.8 g of ketoprofen pure product, which is a white crystal with a melting point of 95 to 96° C. Rate 90%.
Embodiment 2
[0022] Dissolve 23.5g (0.1mol) of 3-nitrile ethyl benzophenone in 100ml of methanol, pour it into a three-necked flask, feed HCl gas, react at room temperature for 2 hours, and add 12g (0.3mol l) NaOH and 100ml of water were refluxed at 70°C for 2 hours. The reaction solution was cooled to room temperature, and the pH value was adjusted to about 3.5 with hydrochloric acid, and a solid was precipitated. Filter through filter paper, dry, recrystallize the filter cake with methanol (300 mL), filter through filter paper, put the filter cake into an oven and dry to obtain 23.6 g of pure ketoprofen, which is a white crystal with a melting point of 95 to 96° C. and a yield of 93%.
Embodiment 3
[0024] Dissolve 23.5g (0.1mol) of 3-nitrile ethyl benzophenone in 100ml of methanol, pour it into a three-necked flask, feed HCl gas, react at room temperature for 1.5 hours, and add 11.2g (0.2mol )KOH and 50ml of water, reflux at 70°C for 2 hours. The reaction solution was cooled to room temperature, and the pH value was adjusted to about 3.5 with hydrochloric acid, and a solid was precipitated. Filter, dry, recrystallize the filter cake with methanol (300 mL), filter, put the filter cake into an oven to dry, and obtain 24.1 g of pure ketoprofen as white crystals, melting point 95-96° C., yield 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com